Dr Clare Materia Medica
Introduction to the Dispensing of Dr Clare’s Blended Herbs
Special | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ALL
B |
|---|
Berberisvulg
Scientific name: Berberis vulgaris Family: Berberidaceae. Traditiona Uses: Orally, the fruit of European barberry is used for kidney, urinary tract, and gastrointestinal tract discomforts such as heartburn, stomach cramps, constipation, lack of appetite, liver and spleen disease, for bronchial and lung discomforts, spasms, as a stimulant for circulation, for people susceptible to infection, and as a supplemental source of vitamin C. The bark, root, and root bark of European barberry are also used orally for ailments and complaints of the GI tract, liver, gallbladder, kidney and urinary tract, respiratory tract, heart and circulatory system, to lower a fever, "blood purifier", and for narcotic withdrawal. European barberry root bark is used for liver dysfunction, gallbladder disease, jaundice, splenopathy, diarrhea, indigestion, hemorrhoids, renal and urinary tract diseases, gout, rheumatism, arthritis, mid and low back pain, malaria, and leishmaniasis. Safety: Traditionally used as a fruit syrup and preserve.when the fruit is consumed orally in food amounts
Evidence from Scientific Research Very little clinical research has been done. Dental Plaque: Preliminary clinical research suggests that brushing with a European barberry extract gel containing 1% berberine three times daily for 3 weeks significantly reduces plaque index compared to placebo and has similar effects compared to a commercial antiplaque toothpaste (Colgate) Diabetes. Small studies suggests that taking European barberry for 8 weeks does not affect blood sugar control in patients with type 2 diabetes How might it work: The applicable parts of European barberry are the fruit (berry), root, bark, and root bark. Preliminary research suggests that European barberry fruit has antihistaminic and anticholinergic effects Preliminary research suggests berberine might inhibit bacterial sortase, a protein responsible for anchoring gram-positive bacteria to cell membranes. Preliminary clinical research suggests it might be useful for topical treatment of burns and trachoma, a common cause of blindness in developing countries. Unwanted effects: Orally, the use of European barberry and other berberine-containing herbs during pregnancy, lactation, or in newborn infants can cause jaundice as the infant have not yet the capacity to metabolise berberine. No adverse reactions have been reported for berberis vulgaris and no clinical studies have been done. In traditional use it is well tolerated. Berberine, a primary constituent of European barberry, has been used orally in adults in doses up to 2 grams/day for 8 weeks with no adverse effects reported Interactions with drugs/supplements: Anticoagulants/antiplatelet drugs: No reported cases of blood clotting problems have been reported with this herb. However Berberine, one of the many constituents of European barberry, may inhibit platelet aggregation in animal studies so caution is advised. Blood sugar control: Clinical study demonstrated no blood sugar lowering effect, but advise monitoring for diabetics as theoretically a lowering of blood sugar is possible. Lowering Blood Pressure: Theoreticall an effect of lowering blood pressure may add to the therapeutic effect of medication, as a precaution remain seated or lie down for 30 minutes after the first 2-3 doses and monitor effect. Transplant antirejection Cyclosporin A (CsA): The constituent Berberine can markedly elevate the blood concentration of CsA in renal-transplant recipients in both clinical and pharmacokinetic studies. This combination may allow a reduction of the CsA dosage. The mechanism for this interaction is most likely explained by inhibition of cytochrome CYP3A4 in the liver and/or small intestine. CYP3A4 Metabolised Drugs: Theoretically blood levels can be elevated or lowered. Common drugs include the SSRI antidepressants, Some Statins (lovastatin), some antibiotics, for full list see http://www.pharmacytimes.com/publications/issue/2008/2008-09/2008-09-8687 Effect on lab. tests: Theoretically, European barberry might increase bilirubin levels. This has been demonstrated with isolated berberine constituent, but not specifically with European barberry. Berberine displaces bilirubin from albumin and increases total and unbound bilirubin concentrations. Effect on conditions or disorders: BLEEDING DISORDERS: Berberine, a constituent of European barberry, might inhibit platelet aggregation. Theoretically, European barberry might increase the risk of bleeding and interfere with therapy in patients with bleeding conditions. Dose: ORAL: No typical dosage. Traditionally, a typical dose is one cup tea.To make tea, steep 1-2 teaspoons of whole or squashed berries in 150 mL boiling water 10-15 minutes and strain or steep 2 grams of root bark in 250 mL boiling water 5-10 minutes and strain. Root bark is typically used as a tincture (1:10), 20-40 drops per day. | |
Black CohoshAlso Known As: Cimicifuga, Scientific Name: Cimicifuga racemosa. Family: Ranunculaceae. People Use This For: Black Cohosh is used for symptoms of menopause, inducing labor in pregnant women, premenstrual syndrome (PMS), painful periods, nervous tension, indigestion, rheumatism, and as a mild sedative. Safety: Possibly safe when used orally and appropriately. Black cohosh has been safely used in some studies lasting up to a year; 1,2,3 There is concern that black cohosh might cause liver damage in some patients. Several case reports link black cohosh to liver failure or autoimmune hepatitis; 4,5,6,7,8,9,10,11,12,13,14,15,16,17 however, there is no conclusive evidence that black cohosh is the cause of liver damage in these patients.18 Until more is known, monitor liver function in patients who take black cohosh for more than three months. Analysis of 9 Cases of Suspected association between Black Cohosh and Hepatitis concluded that there is little if any hepatotoxic risk by the use of Black Cohosh in these cases. Menopause 2009 Sep-Oct: 16(5):956-65 Teschke R, Bahre R, Fuchs J, Wolff A. It is concluded that the use of BC may not exert an overt toxicity risk, but quality problems in a few BC products were evident that require additional regulatory quality specifications. Ann Hepatology. 2011 Jul-Sep;10(3):249-59 Pregnancy and Lactation: Refer to Medical Herbalist Effectiveness: POSSIBLY EFFECTIVE Menopausal symptoms. Some black cohosh extracts seem to modestly reduce symptoms of menopause, such as hot flashes. However, there is considerable variability in the preparations used in clinical trials, and in the results obtained.19 INSUFFICIENT RELIABLE EVIDENCE to RATE Osteoporosis: Preliminary clinical research suggests that postmenopausal women who take a specific black cohosh extract CR BNO 1055 (Klimadynon/Menofem, Bionorica AG) 40 mg/day have increased levels of bone-specific alkaline phosphatase (bALP), which is a marker of bone formation, after 12 weeks of treatment. 20 However, it is not known if this black cohosh extract can increase bone mineral density or decrease the risk of fracture. More evidence is needed to rate black cohosh for these uses. Mechanism of Action: The applicable parts of black cohosh are the rhizome and root. The active constituents of black cohosh include phytosterin; isoferulic acid; fukinolic acid; caffeic acid; salicylic acid; sugars; tannins; long-chain fatty acids; and triterpene glycosides, including acetein, cimicifugoside, and 27-deoxyactein.21,22 Black cohosh has estrogen-like effects (not estrogenic effects) that are exerted by an unknown mechanism.21,23,20 Adverse Reactions: Black cohosh can commonly cause gastrointestinal upset.4,24,25 Black cohosh has associated with weakness and muscle damage in one case. In another case, a single patient developed symptoms of cutaneous pseudolymphoma 6 months after starting a specific black cohosh extract (Remifemin). Symptoms resolved within 12 weeks of discontinuing black cohosh.26 There are two case reports of cutaneous vasculitis in menopausal women who took black cohosh-containing products. In these cases, both women were taking a combination product containing black cohosh 40 mg (Estroven, Amerifit Brands). Symptoms resolved within 3 months of discontinuing the product.27 Interactions with Herbs & Supplements: Refer to a Medical Herbalist. Interactions with Drugs: Atorvastatin (Lipitor) One report of significant interaction. Chemotherapy: Refer to a Medical Herbalist. Hepatotoxic Drugs: Refer to a Medical Herbalist. Interactions with Foods: None known. Interactions with Lab Tests: Liver Function Tests: Elevated liver function tests have not been documented in clinical trials.20 Theoretically, some patients taking black cohosh might experience elevated liver function tests. Interactions with Diseases or Conditions: Breast Cancer: Refer to Medical Herbalist. Hormone-Sensitive Cancers/Conditions: Black cohosh doesn't seem to affect estrogen receptors. Refer to Medical Herbalist. Kidney Transplant: Refer to Medical Herbalist. Liver Disease: Refer to Medical Herbalist. Dosage/Administration: Dr Clare’s Blend: 1gm/day Oral: 0.5-1gms/day Specific References: BLACK COHOSH 1. Raus K, Brucker C, Gorkow C, Wuttke W. First-time proof of endometrial safety of the special black cohosh extract (Actaea or Cimicifuga racemosa extract) CR BNO 1055. Menopause 2006;13:678-91. Newton KM, Reed SD, LaCroix AZ, et al. Treatment of vasomotor symptoms of menopause 2. with black cohosh, mulitbotanicals, soy, hormone therapy, or placebo. Ann Intern Med 2006;145:869-79. Available at: http://www.annals.org/cgi/reprint/145/12/869.pdf. 3. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause 2009;16:1156-66. 4. Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust 2002;177:440-3. 5. Lontos S, Jones RM, Angus PW, Gow PJ. Acute liver failure associated with the use of herbal preparations containing black cohosh. Med J Aust 2003;179:390-1. 6. Cohen B, Schardt D. Center for Science in the Public Interest. Letter to Food and Drug Administration. Commissioner Mark McClellan, MD, PhD. March 4, 2004. Cohen SM, O'Connor AM, Hart J, et al. Autoimmune hepatitis associated with the use of 7. black cohosh: a case study. Menopause 2004;11:575-7. 8. Levitsky J, Alli TA, Wisecarver J, Sorrell MF. Fulminant liver failure associated with the use of black cohosh. Dig Dis Sci 2005;50:538-9. 9. MHRA. Black cohosh (Cimicifuga racemosa) - risk of liver problems. Herbal Safety News IdcService=SS_GET_PAGE&useSecondary= true&ssDocName=CON2024131&ssTargetNodeId=663. 10. Lynch CR, Folkers ME, Hutson WR. Fulminant hepatic failure associated with the use of black cohosh: a case report. Liver Transpl 2006;12:989-92. 11. Chow ECY, Teo M, Ring JA, Chen JW. Liver failure associated with the use of black cohosh for menopausal symptoms. Med J Aust 2008;188:420-2. 12. Mahady GB, Low Dog T, Barrett ML, et al. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008;15:628-38. 13. Hepatotoxicity with black cohosh. Australian Adv Drug Reactions Bull 2006;25:6. Available at: www.tga.gov.au/adr/aadrb/aadr0604.htm#a1. 14. Dunbar K, Solga SF. Black cohosh, safety, and public awareness. Liver Int 2007;27:1017. 15. Patel NM, Derkits RM. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Pract 2007;20:341-6. 16. Joy D, Joy J, Duane P. Black cohosh: a cause of abnormal postmenopausal liver function tests. Climacteric 2008;11:84-8. 17. Australian Adverse Drug Reactions Advisory Committee. Black cohosh and liver toxicity - an update. Aust Adv Drug Reactions Bull 2007;26:11. 18. Teschke R, Bahre R, Genthner A, et al. Suspected black cohosh hepatotoxicity - challenges July 2006. Available at: http://www.mhra.gov.uk/home/idcplg? and pitfalls of causality assessment. Maturitas 2009;63:302-14. 19. Shams T, Setia MS, Hemmings R, et al. Efficacy of black cohosh-containing preparations on menopausal symptoms: a meta-analysis. Altern Ther Health Med 2010;16:36-44. 20. Wuttke W, Gorkow C, Seidlova-Wuttke D. Effects of black cohosh (Cimicifuga racemosa) on bone turnover, vaginal mucosa, and various blood parameters in postmenopausal women: a double-blind, placebo-controlled, and conjugated estrogens-controlled study. Menopause 2006;13:185-96. 21. Kruse SO, Lohning A, Pauli GF, et al. Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med 1999;65:763-4. 22. Loser B, Kruse SO, Melzig MF, Nahrstedt A. Inhibition of neutrophil elastase activity by cinnamic acid derivatives from Cimicifuga racemosa. Planta Med 2000;66:751-3. Einer-Jensen N, Zhao J, Andersen KP, Kristoffersen K. Cimicifuga and Melbrosia lack 23. oestrogenic effects in mice and rats. Maturitas 1996;25:149-53. 24. Pepping J. Black cohosh: Cimicifuga racemosa. Am J Health Syst Pharm 1999;56:1400-2. 25. Liske E. Therapeutic efficacy and safety of Cimicifuga racemosa for gynecologic disorders. Adv Ther 1998;15:45-53. 26. Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology 2007;214:94-6. 27. Ingraffea A, Donohue K, Wilkel C, Falanga V. Cutaneous vasculitis in two patients taking an herbal supplement containing black cohosh. J Am Acad Dermatol 2007;56:S124-6. | |
Bladderwrack
Scientific Name: Fucus vesiculosus; Ascophyllum nodosum; other Fucus species. Family: Fucaceae. People Use This For: Bladderwrack is used for thyroid disorders, iodine deficiency, goiter, obesity, arthritis, and rheumatism, "blood cleansing", to increase energy, constipation, bronchitis, decreased resistance to disease, and anxiety. Topically, bladderwrack is used for skin diseases, burns, aging skin, and insect bites. Safety: No concerns regarding safety when used orally in appropriate doses. It is important to obtain traceable supply free from contamination (1 case of contamination with heavy metals reported in the 1970’s).1,2 Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Obesity: Preliminary clinical research suggests that bladderwrack in combination with lecithin and vitamins doesn't result in sustained weight loss. More evidence is needed to rate bladderwrack for this use (combined lecithin, kelp, multivitamin preparation involving 120 women over 2 years). 3 Mechanism of Action: The applicable part of bladderwrack is the entire plant. Bladderwrack is a brown seaweed. Bladderwrack contains high concentrations of iodine, which is present in varying amounts. Bladderwrack is a source of fiber, minerals such as iron, and vitamin B12.2 Preliminary clinical research suggests bladderwrack may normalize the menstrual cycle and have estrogen balancing effects in premenopausal women. It may also balance progesterone effects4 (case reports on three patients). Preliminary clinical research suggests topical administration of bladderwrack extract might reduce skin thickness and other signs of aging.5 Adverse Reactions: Excess Iodine intake is rare in humans outside of radiation contamination or excessive amounts of seaweed (or seaweed extracts) intake over a prolonged period. There is one case report of heavy metal poisoning where arsenic poisoning occurred with ingestions of a contaminated kelp product. 6 Another case of arsenic-related poisoning with bladderwrack ingestion 400 mg three times a day for 3 months resulted in kidney damage.7 Interactions with Herbs & Supplements: Avoid Iodine supplements at the same time. Interactions with Drugs: Antithyroid Drugs: Theoretically, may result in additive hypothyroid activity, and may lower the level of availableThyroid hormones.8 Interactions with Foods: None known. Interactions with Lab Tests: Radioactive Iodine Uptake: Theoretically, bladderwrack might interfere with the results of thyroid function tests using radioactive iodine uptake.2 Thyroid Stimulating Hormone (TSH): Theoretically, bladderwrack might increase serum TSH levels and test results.8 Thyroxine (T4): Theoretically, bladderwrack might increase serum T4 levels and test results.8 Interactions with Diseases or Conditions: Iodine Allergy: Avoid bladderwrack use in people sensitive to iodine.8 Thyroid Disorders: Prolonged use or excessive amounts of iodides may exacerbate thyroid gland problems.8 Dosage/Administration: Dr Clare’s Blends: 1 gm per day No typical dosage. Specific References: BLADDERWRACK 1. Baker DH. Iodine toxicity and its amelioration. Exp Biol Med (Maywood) 2004;229:473-8. 2. Phaneuf D, Cote I, Dumas P, et al. Evaluation of the contamination of marine algae (Seaweed) from the St. Lawrence River and likely to be consumed by humans. Environ Res 1999;80:S175-S182. 3. Bjorvell H, Rössner S. Long-term effects of commonly available weight reducing programmes in Sweden. Int J Obes 1987;11:67-71. 4. Skibola CF. The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: a case report. BMC Complement Altern Med 2004;4:10. 5. Fujimura T, Tsukahara K, Moriwaki S, et al. Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. J Cosmet Sci 2002;53:1- Pye KG, Kelsey SM, House IM, et al. Severe dyserythropoeisis and autoimmune 6. thrombocytopenia associated with ingestion of kelp supplement. Lancet 1992;339:1540. 7. Conz PA, La Greca G, Benedetti P, et al. Fucus vesiculosus: a nephrotoxic alga? Nephrol Dial Transplant 1998;13:526-7. 8. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2002. Available at: www.nap.edu/books/0309072794/html/. | |
Boneset
Agueweed, Crosswort, Eupatoire, Eupatorio, Feverwort, Indian Sage, Sweating Scientific Name: Eupatorium perfoliatum. Family: Asteraceae/Compositae. People Use This For: Orally, boneset is used as an antipyretic, diuretic, laxative, emesis, and cathartic. Safety: POSSIBLY UNSAFE: When used orally in excessive amounts. Large doses are both cathartic and emetic. Though the alkaloids have not been characterized, hepatotoxic pyrrolizidine alkaloids (PAs) are common in this genus (3). PREGNANCY AND LACTATION: POSSIBLY UNSAFE ...when used orally, due to possible hepatotoxic pyrrolizidine alkaloid content (3); avoid using. Effectiveness: There is insufficient reliable information available about the effectiveness of boneset. Mechanism of Action: The applicable parts of boneset are the dried leaf and flowering parts. Preliminary research suggests boneset might have cytotoxic and mild antibacterial activity (4). Adverse Reactions: Report an Adverse Reaction to BONESET Orally, boneset can cause an allergic reaction in individuals sensitive to the Asteraceae / Compositae family. Members of this family include ragweed, chrysanthemums, marigolds, daisies, and many other herbs. Interactions with Herbs & Supplements: HEPATOTOXIC : Concomitant use is contraindicated due to the risk of additive toxicity. Herbs containing hepatotoxic PAs include borage, butterbur, coltsfoot, comfrey, gravel root, hemp agrimony, hound's tongue, and the Senecio species plants dusty miller, alpine ragwort, groundsel, golden ragwort, and tansy ragwort (2). PYRROLIZIDINE ALKALOID (PA)-CONTAINING Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: CROSS-ALLERGENICITY: Boneset can cause an allergic reaction in individuals sensitive to the Asteraceae/Compositae family. Members of this family include ragweed, chrysanthemums, marigolds, daisies, and many other herbs. Dosage/Administration: ORAL: Traditionally one cup of tea, prepared by steeping 1-2 grams herb in 150 mL boiling water, has been used three times daily. The liquid extract, 1:1 in 25% alcohol, has been used 1-2 mL three times daily. 1-4 mL of the tincture, 1:5 in 45% alcohol, has also been used three times daily (1). Specific References: THYME 1. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare 2. Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine 3. Roeder E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 4. Habtemariam S, Macpherson AM. Cytotoxicity and antibacterial activity of ethanol extract from leaves of a herbal drug, boneset (Eupatorium perfoliatum). Phytother Res 2000;14:575-7. | |
Boswellia
Also known as: Indian Frankincense, Indian Olibanum, Ru Xiang, Salai Guggul. Scientific name: Boswellia serrata. Botanical Family: Burseraceae. Part used: The medicinal part of Indian frankincense plant is the gum resin. The gum resin is obtained by pulling away the bark of the Indian frankincense tree. Traditional use. Indian frankincense is used for osteoarthritis, rheumatoid arthritis, rheumatism, bursitis, and tendonitis. Other uses include ulcerative colitis, abdominal pain, asthma, allergic rhinitis, sore throat, painful menstruation, acne, and cancer. It is also used as a stimulant, respiratory antiseptic, diuretic, and for stimulating menstrual flow. Safety. There are no safety concerns when used orally and appropriately. Indian frankincense has been safely used in several clinical trials lasting up to ninety days. (1,2, 3, 4, 5, 6, 7, 8, 9, 10) Pregnancy: Safe when used orally in amounts commonly found in foods.(11) For medicinal use consult an Herbal Medicine Physician. Breastfeeding: Safe when used orally in amounts commonly found in foods.(11) For medicinal use consult an Herbal Medicine Physician. Constituents Boswellic acids (triterpenoids) Essential oils including cymene and thujone Scientific evidence. Osteoarthritis. Some clinical research shows that taking specific Indian frankincense extracts can reduce symptoms of osteoarthritis. In two clinical trials, using a specific Indian frankincense extract (5-Loxin) 100 mg daily or 250 mg daily significantly improved pain and functionality scores in patients with osteoarthritis after 90 days of treatment. Pain scores were reduced by about 32% to 65%. Patients began to have significant improvement within 7 days of treatment. The extract used in this study was standardized and enriched to contain 30% of the boswellic acid AKBA. (8,9) One clinical trial evaluated another specific Indian frankincense extract (Aflapin) 100 mg daily. This extract significantly improved pain and functionality scores in patients with osteoarthritis after 90 days of treatment. Pain scores were reduced by about 47%. Patients began to have significant improvement within 7 days of treatment. The extract used in this study was standardized and enriched to contain 20% of the boswellic acid AKBA. (9) In a preliminary crossover trial, taking a different Indian frankincense extract, 333mg daily also significantly reduced symptoms of osteoarthritis, such as knee pain and swelling. (3) Ulcerative colitis. Two clinical trials show that taking Indian frankincense can improve some symptoms of ulcerative colitis and some pathological measures. In one study, taking whole plant Indian frankincense resin 350 mg three times daily significantly improved symptoms and disease markers in patients with ulcerative colitis. In this study, about 82% of patients taking Indian frankincense went into remission compared to 75% taking the commonly prescribed drug sulfasalazine.(2) In another preliminary clinical study, taking whole plant Indian frankincense resin 300 mg three times for 6 weeks improved symptoms and some measures of disease pathology in about 90% of patients. In this study 70% of patients taking Indian frankincense went into remission compared to 40% taking sulfasalazine 3 grams daily. (7) Asthma. There is some preliminary evidence that taking Indian frankincense extract orally might help asthma. It may improve forced expiratory volume, reduce the number of asthma attacks, and decrease clinical signs of asthma. (1) Crohn's disease. There is preliminary evidence that taking Indian frankincense extract orally might reduce some symptoms of Crohn’s disease. One clinical study found that it worked as well as mesalamine (Asacol, Pentasa) for Crohn's disease;(6) however, other clinical research shows that taking Indian frankincense 800 mg orally three times a day did not increase rates of remissions and quality of life any more than placebo in patients with Crohn's disease. (12) Rheumatoid arthritis. There is conflicting research about the usefulness of Indian frankincense extract taken orally for rheumatoid arthritis. (4,5) Mechanism of action. The principle constituents of Indian frankincense are boswellic acid and alpha- and beta-boswellic acid, which are thought to have anti-inflammatory properties. (13,14) The boswellic acid (AKBA) constituent appears to be the most potent anti-inflammatory constituent. (14) The gum resin also contains up to 16% essential oils including alpha-thujene and p-cymene. (15) In preliminary research, some Indian frankincense extracts show anti-inflammatory, analgesic, and anti-arthritis effects; however, not all Indian frankincense-containing products seem to have these effects. (3) Boswellic acids, especially AKBA, inhibit 5-LOX enzymes and reduce leukotriene synthesis and inhibit leukocyte elastase, which are the likely mechanisms for its anti-inflammatory properties. Boswellic acids may also have a disease modifying effect by decreasing the breakdown of glycosaminoglycan and thus reducing cartilage damage. Indian frankincense may also inhibit mediators of autoimmune disorders. It seems to reduce production of antibodies and cell-mediated immunity. (3,7,16,17) Indian frankincense may be useful in treating cancer. Preliminary research suggests that boswellic acids have an anti-proliferative effect and an effect of increasing the rate of cell death (apoptosis) in cancer cells. (16) Preliminary research suggests that boswellic acids stabilize mast cells, this suggests usefulness for asthma and other allergic conditions.(18) Other preliminary research suggests that boswellic acids might help prevent organ rejection and ischemia/reperfusion injury.(19) Indian frankincense has an elimination half-life of 6 hours from the blood stream.(20) Adverse reactions. Indian frankincense is well-tolerated. Side effects reported in clinical trials did not occur more commonly than placebo. (8) Some reported side effects include diarrhea, nausea, abdominal pain, and heartburn. (3,7,8 ,9 ,10) No serious adverse events have been documented.(10) Interactions with herbs and supplements. None known. Interactions with drugs. None known. Interactions with foods. None known. Interactions with lab tests. None known. Interactions with diseases or conditions. None known. Dosage. For osteoarthritis, a specific Indian frankincense extract (5-Loxin) 100 mg daily or 250 mg daily has been used.(8) Indian frankincense extract 333 mg three times daily has been used. (3) For rheumatoid arthritis, Indian frankincense extract 3,600 mg daily has been used. (4) For Crohn's disease, 800 mg three times daily has been used. (21) For ulcerative colitis, a gum resin preparation of 300-350 mg three times daily has been used. (2,7) For asthma, 300 mg three times daily has been used. (1) | |
Buchu
Barosmae Folium. Scientific Name: Barosma betulina People Use This For: Orally, buchu is used as a urinary tract disinfectant in cystitis, urethritis, prostatitis, benign prostatic hyperplasia and kidney infections. In manufacturing, the oil from buchu is used to give a fruit flavor (often black currant) to foods. Safety: No concerns regarding safety, available studies validate this statement, when the leaf is used in amounts commonly found in foods. Buchu has Generally Recognized As Safe status (GRAS) for use in foods in the US.1 No converns regarding safety when the leaf is used orally and appropriately in medicinal amounts.2,3 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: Not enough scientific information gathered to offer a comment Mechanism of Action: The applicable part of buchu is the leaf. Buchu camphor (also known as diosphenol) is the principal constituent of the oil. Researchers believe this constituent may be responsible for buchu's reported diuretic and antiseptic effects.4 Adverse Reactions: Occasional digestive upset if taken on an empty stomach.R1 pp.311 Interactions with Herbs & Supplements: None Interactions with Drugs: Lithium: Because of diuretic effect.R3 pp.163-164 Diuretics: Effect can be additive.R4 pp.192,204,215 Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: Surgery: Tell patients to discontinue buchu at least 2 weeks before elective surgical procedures. Dosage/Administration: Oral: The typical dose is 1 cup of tea (steep 1 gram dry leaf in 150 mL boiling water 5-10 minutes, strain) several times per day.5 Specific References: BUCHU 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 2. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 3. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 4. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 5. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. Stuttgart: Medpharm GmbH Scientific Publishers, 1994. | |
Burdock Root
Arctium, Beggar's Buttons, Burr Seed, Clotbur, Cocklebur. Scientific Name: Arctium lappa; Arctium minus; Arctium tomentosum. Family: Asteraceae/Compositae. People Use This For: Burdock is used as a diuretic, "blood purifier", antimicrobial, and an antipyretic. It is also used to treat gastrointestinal complaints, rheumatism, gout, cystitis, and chronic skin conditions including acne and psoriasis. It is also used for hypertension, arteriosclerosis, hepatitis, and other inflammatory conditions. SourceURL:file://localhost/Users/ruthruane/Downloads/Herbal%20Medicine-Newest.doc Burdock is also used for treating colds, catarrh. Topically, burdock is used for dry skin, acne, psoriasis, and eczema. The root of burdock is consumed as a food. Safety: No concerns regarding safety when used in amounts commonly found in foods.9,10 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available to comment on the effectiveness of burdock. Mechanism of Action: The applicable relevant part of burdock is the root. Extracts of burdock root appear to have cough suppressant activity and may increase immunological activity.11 Other preliminary research suggests it might have anti-inflammatory and free radical scavenging activity.12 Burdock root extract might also protect the liver from toxicity caused by ethanol and carbon tetrachloride, possibly due to its antioxidant activity.9 Adverse Reactions: An isolated report of an allergic reaction causing anaphylaxis.10 Interactions with Foods: None known. Interactions with Lab Tests: SourceURL:file://localhost/Users/ruthruane/Downloads/Herbal%20Medicine-Newest.doc None known. Dosage/Administration: No typical dosage Specific References: BURDOCK 9. Lin SC, Lin CH, Lin CC, et al. Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J Biomed Sci 2002;9:401-9. 10. Sasaki Y, Kimura Y, Tsunoda T, Tagami H. Anaphylaxis due to burdock. Int J Dermatol 2003;42:472-3. 11. Kardosova A, Ebringerova A, Alfoldi J, et al. A biologically active fructan from the roots of Arctium lappa L., var. Herkules. Int J Biol Macromol 2003;33:135-40. 12. Lin CC, Lu JM, Yang JJ, et al. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am J Chin Med 1996;24:127-37. | |

 Also known as
Also known as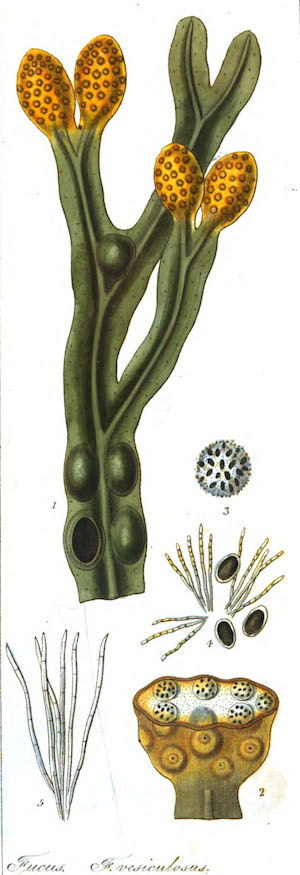 Also Known As: Fucus, Kelp, Knotted Wrack.
Also Known As: Fucus, Kelp, Knotted Wrack. Also Known As:
Also Known As: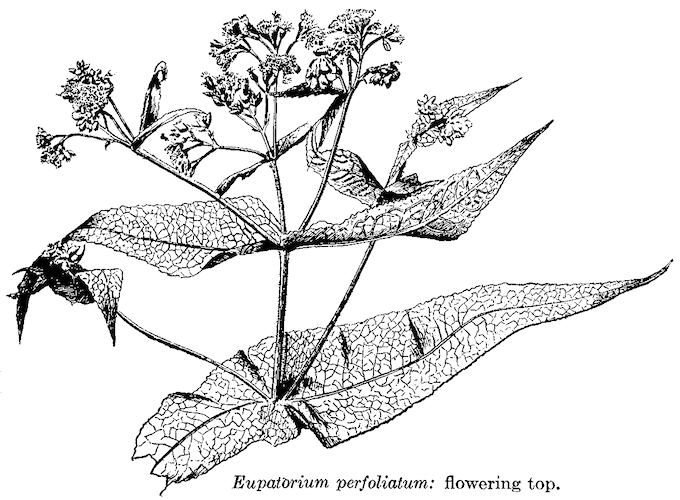 Also Known As:
Also Known As: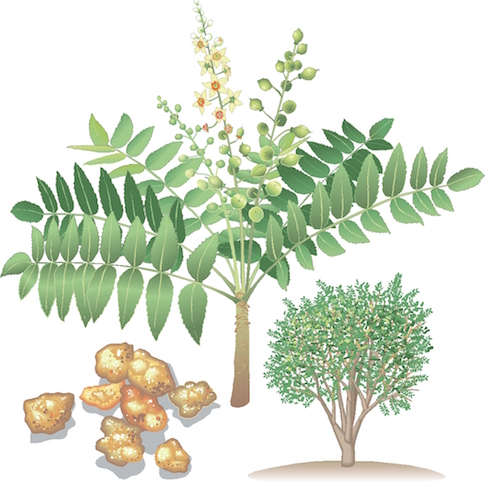 Boswellia
Boswellia Also Known As:
Also Known As: Also Known As:
Also Known As: