Dr Clare Materia Medica
Introduction to the Dispensing of Dr Clare’s Blended Herbs
Special | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ALL
H |
|---|
HorsetailHorsetail
Scientific Name: Equisetum arvense; Equisetum telmateia, Equisetum hyemale. Family: Equisetaceae.
People Use This For: Horsetail is used for diuresis, edema, kidney and bladder stones, urinary tract infections, incontinence and general disturbances of the kidney and bladder. It is also used for hair loss; tuberculosis;jaundice; hepatitis; brittle fingernails; rheumatic diseases; gout; osteoarthritis; osteoporosis; frostbite; weight loss; menorrhagia; and nasal, pulmonary, and gastric hemorrhage. Topically, horsetail is used for treatment of wounds and burns.
Safety: POSSIBLY UNSAFE ...when used orally long-term. Horsetail contains thiaminase, an enzyme that can cause thiamine deficiency. In Canada, horsetail products are required to be thiaminase-free, but there is not enough reliable information to know if thiaminase-free products are safe (3). PREGNANCY AND LACTATION: Insufficient reliable information available; avoid using.
Scientific evidence: Not enough scientific research has been done on Horsetail to comment on effectiveness.
Mechanism of Action: The applicable parts of horsetail are above ground parts. Horsetail constituents include flavonoids such as apigenin, luteolin, and kaempferol and quercetin compounds; petrosins such as onitin; caffeic acid derivatives; sterols; tannins; and saponins (6, 7, 9). Horsetail also contains significant amounts of silicon (4). Horsetail also contains trace amounts of nicotine (4). Preliminary research suggests aqueous and hydroalcoholic extracts of horsetail have antioxidant and anti-inflammatory effects (8, 10,11). Horsetail extracts might also have vasorelaxant and analgesic effects (7, 11). Other research suggests horsetail extracts might have sedative and anticonvulsant effects (8). Flavonoid and petrosin constituents might have hepatoprotective properties (6). Other preliminary research suggests that horsetail might have antiviral effects (12). Crude horsetail contains thiaminase, an enzyme that destroys thiamine (vitamin B1). Thiaminase-containing plants have been associated with neurological toxicity in animals due to thiamine deficiency (12, 14, 15). Other related horsetail (Equisetum) species have diuretic and hypoglycemic properties (16, 17, 18). Whether horsetail has these effects is unclear.
Side effects: Crude horsetail may lead to thiamine deficiency with prolonged consumption. Canadian products are required to be certified as free from thiaminase-like effect (3). Horsetail has also been associated with cross allergenicity with carrots (19). Horsetail contains tiny amounts of nicotine and may cause nicotine allergy or theoretically, nicotine toxicity if taken in large quantities (5). Topically, horsetail can cause seborrheic dermatitis (5).
Interactions with Herbs & Supplements: Betel nuts: Consuming horsetail with betel nuts might increase the risk of thiamine deficiency. Areca (betel) nuts reduce thiamine activity, probably by chemical inactivation (2). Horsetail contains thiaminase, which breaks down thiamine (13, 14, 15). CHROMIUM-CONTAINING HERBS AND SUPPLEMENTS: Horsetail contains chromium (0.0006%) and could increase the risk of chromium toxicity when taken with chromium supplements or chromium-containing herbs such as bilberry, brewer's yeast, or cascara (1). THIAMINE: Crude horsetail contains thiaminase, which breaks down thiamine. Chronic ingestion in animals has been associated with thiamine deficiency (13, 14, 15). Possible Interactions with Drugs: LITHIUM In keeping with all herbs and medications that have a diuretic action the blood levels of Lithium may be sensitive to the diuretic effect. Horsetail is thought to have diuretic properties. Theoretically, due to these potential diuretic effects, horsetail might reduce excretion and increase levels of lithium. The dose of lithium might need to be decreased.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: Diabetes: Other horsetail species have hypoglycemic activity (18). It is unclear whether horsetail has hypoglycemic effects. Until more is known, bear this in mind when using in the context of diabetes. Monitor blood sugar levels to assess any effects. Low blood potassium: Other horsetail species have diuretic activity and can increase the excretion of potassium (16, 17). Until more is known, use with caution in patients who are at risk for potassium deficiency. Thiamine deficiency: Theoretically, horsetail can cause or exacerbate thiamine deficiency (13, 14, 15). No cases have been reported in humans. For every three continuous use take a break for three weeks.
Dosage/Administration: No typical dosage.
Specific References: HORSETAIL 1. Lanca S, Alves A, Vieira AI, et al. Chromium-induced toxic hepatitis. Eur J Intern Med 2002;13:518-20. 2. Vimokesant S, Kunjara S, Rungruangsak K, et al. Beriberi caused by antithiamin factors in food and its prevention. Ann N YAcad Sci 1982;378:123-36. 3. Health Canada. Labelling Standard: Mineral Supplements. Available at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/label-etiquet-pharm/minsup_e.html (Accessed 14 November 2005). 4. Piekos R, Paslawska S. Studies on the optimum conditions of extraction of silicon species from plants with water. I. Equisetum arvense L. Herb. Planta Med 1975;27:145-50. 5. Sudan BJ. Seborrhoeic dermatitis induced by nicotine of horsetails (Equisetum arvense L.). Contact Dermatitis 1985;13:201-2. 6. Oh H, Kim DH, Cho JH, Kim YC. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J Ethnopharmacol 2004;95:421-4. 7. Sakurai N, Iizuka T, Nakayama S, et al. [Vasorelaxant activity of caffeic acid derivatives from Cichorium intybus and Equisetum arvense]. Yakugaku Zasshi 2003;123:593-8. 8. Dos Santos JG Jr, Blanco MM, Do Monte FH, et al. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia 2005;76:508-13. 9. Langhammer L, Blaszkiewitz K, Kotzorek I. Evidence of toxic adulteration of equisetum. Dtsch Apoth Ztg 1972;112:1751-94. 10. Correia H, Gonzalez-Paramas A, Amaral MT, et al. Characterisation of polyphenols by HPLC-PAD-ESI/MS and antioxidant activity in Equisetum telmateia. Phytochem Anal 2005;16:380-7. 11. Do Monte FH, dos Santos JG Jr, Russi M, et al. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol Res 2004;49:239-43. 12. Husson GP, Vilagines R, Delaveau P. [Antiviral properties of various extracts of natural origin]. Ann Pharm Fr 1986; 44:41-8. 13. Ramos JJ, Ferrer LM, Garcia L, et al. Polioencephalomalacia in adult sheep grazing pastures with prostrate pigweed. Can Vet J 2005;46:59-61. 14. Henderson JA, Evans EV, McIntosh RA. The antithiamine action of Equisetum. J Am Vet Med Assoc 1952;120:375-8. 15. Fabre B, Geay B, Beaufils P. Thiaminase activity in equisetum arvense and its extracts. Plant Med Phytother 1993;26:190-7. 16. Perez Gutierrez RM, Laguna GY, Walkowski A. Diuretic activity of Mexican equisetum. J Ethnopharmacol 1985;14:269-72. 17. Lemus I, Garcia R, Erazo S, et al. Diuretic activity of an Equisetum bogotense tea (Platero herb): evaluation in healthy volunteers. J Ethnopharmacol 1996;54:55-8. 18. Revilla MC, Andrade-Cetto A, Islas S, Wiedenfeld H. Hypoglycemic effect of Equisetum myriochaetum aerial parts on type 2 diabetic patients. J Ethnopharmacol 2002;81:117-20. 19. Agustin-Ubide MP, Martinez-Cocera C, Alonso-Llamazares A, et al. Diagnostic approach to anaphylaxis by carrot, related vegetables and horsetail (Equisetum arvense) in a homemaker. Allergy 2004;59:786-7. | |
HyssopHyssop
Hysope Officinale.
Scientific Name: Hyssopus officinalis. Family: Lamiaceae/Labiatae.
People Use This For: Hyssop is used for liver and gallbladder conditions, intestinal inflammation, coughs, the common cold, respiratory infections, sore throat, asthma, urinary tract infection, flatulence and colic, anorexia, poor circulation, painful periods, and for digestive and intestinal problems.
Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Generally Recognized as Safe (GRAS) status in the US.(12) Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: There is insufficient scientific information available about the effectiveness of hyssop.
Mechanism of Action: The applicable parts of hyssop are the above ground parts. Constituent marrubiin (13) has cardioactive effects and stimulates bronchial secretions. (14) Caffeic acid and tannins may be the active constituents of the dried leaves. Extracts show antiviral activity against herpes simplex virus and HIV in vitro. (15,13)
Adverse Reactions: None reported with tincture or infusion.
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: None known.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: None reported with tincture or infusion.
Dosage/Administration: Dr Clare’s Blends: To check Oral: Typically people take two 445 mg capsules containing the hyssop herb three times daily. (16) Some people take 10-15 drops of the hyssop extract (12-14% by volume) in water two to three times daily. (17) People also consume or gargle the hyssop tea three times daily. (18) The tea is prepared by steeping 1-2 teaspoons of the dried hyssop flower tops in 150 mL boiling water for 10-15 minutes and then straining. Avoid internal use of hyssop oil due to possible neurotoxicity.
Specific References: HYSSOP 12. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 13. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. 14. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 15. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 16. Manufacturer: Nature's Way. Springville, UT. 17. Manufacturer: Nature's Answer. Hanppange, NY. 18. Fetrow CW, Avila JR. Professional's Handbook of Complementary & Alternative Medicines. 1st ed. Springhouse, PA: Springhouse Corp., 1999. | |

 Also Known As:
Also Known As: 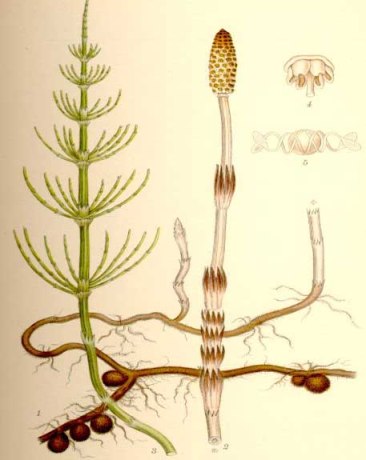 Also
Known As: Bottle Brush, Common Horsetail, Corn Horsetail, Dutch Rushes,
Equiseti Herba, Field Horsetail, Herbe à Récurer, Horse Herb, Horse Willow,
Paddock-Pipes, Pewterwort, Scouring Rush, Shave Grass, Spring Horsetail, Toadpipe.
Also
Known As: Bottle Brush, Common Horsetail, Corn Horsetail, Dutch Rushes,
Equiseti Herba, Field Horsetail, Herbe à Récurer, Horse Herb, Horse Willow,
Paddock-Pipes, Pewterwort, Scouring Rush, Shave Grass, Spring Horsetail, Toadpipe. Also Known As:
Also Known As: