Dr Clare Materia Medica
Introduction to the Dispensing of Dr Clare’s Blended Herbs
Special | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ALL
A |
|---|
AshwagandaAlso Known As: Withania. Scientific Name: Withania somnifera. Family: Solanaceae. People Use This For: Ashwagandha is traditionally used for arthritis, anxiety, insomnia, tumors, tuberculosis, and chronic liver disease. Ashwagandha is also used as an "adaptogen" to increase resistance to environmental stress, and as a general tonic. It is also used for immunomodulatory effects, improving cognitive function, decreasing inflammation, preventing the effects of aging, for emaciation, infertility in men and women, menstrual disorders, fibromyalgia, and hiccups. It is also used orally as an aphrodisiac. Safety: Possibly safe when used orally and appropriately, short-term.17,18 Pregnancy and Breastfeeding: Insufficient scientific information available, consult a medical herbalist. Effectiveness: There is insufficient scientific information available to comment. Mechanism of Action: The applicable parts of ashwagandha are the root and berry. Ashwagandha contains several active constituents including alkaloids, steroidal lactones, and saponins.19,18. Animal model research suggests that ashwagandha has a variety of pharmacological effects including pain relief, lowering temperature, reducing anxiety, inflammatory, and antioxidant effects.17,20,21,19,18 , sedative, blood pressure lowering, anti-immunomodulatory. Some researchers think ashwagandha has a so-called "anti-stressor" effect. Preliminary increases of dopamine receptors in the corpus striatum of the brain. 17 It also appears to reduce stress-induced increases of plasma corticosterone, blood urea nitrogen, and blood lactic acid.18 Ashwagandha seems to have anxiolytic effects, possibly by acting as a gamma-aminobutyric acid (GABA) mimetic agent. Research suggests ashwagandha suppresses stress-induced anxiety. Ashwagandha and its constituents also seem to have modulating effects on the immune system. The withanolides and sitoindosides seem to cause a mobilization of phagocytosis, and lysosomal enzymes.18 Adverse Reactions: Ashwagandha is well tolerated. Interactions with Herbs & Supplements Herbs and Supplements with Sedative Properties: Theoretically, used with herbs that have sedative properties they may have an additive effect. This needs to be taken into account with the dosage. Interactions with Drugs: Benzodiazepines e.g.Valium, Xanax CNS Depressants: Theoretically, Ashwagandha's sedative effect may add to the effects of barbiturates (rarely prescribed now except for epilepsy), other sedatives, and drugs for anxiety,17 needed. Immunosuppressants; refer to medical herbalist Thyroid Hormone: Theoretically, ashwagandha might have additive effects when used with thyroid supplements. There is preliminary evidence that ashwagandha might boost thyroid hormone synthesis and/or secretion.17 Refer patients on medication to a well qualified Medical Herbalist. Interactions with Foods: None known. Interactions with Lab Tests: Digoxin blood levels (heart medication). Interactions with Diseases or Conditions: Autoimmune effects.17,20,22,18 The modulating effects on the immune system can be helpful but should be prescribed by a Medical Herbalist. Diseases: Ashwagandha may have immunostimulant properties. Dosage/Administration: Dr Clare’s Blends: 1gm per day Oral: People typically use 1 to 6 grams daily of the whole herb in capsule or tea form.17 The tea is prepared by boiling ashwagandha roots in water for 15 minutes and cooled. The usual dose is 3 cups daily. Tincture or fluid extracts are dosed 2 to 4 mL 3 times per day. Topical: No typical dosage. Specific References: ASHWAGANDHA Upton R, ed. Ashwagandha Root (Withania somnifera): Analytical, quality control, and 17. therapuetic monograph. Santa Cruz, CA: American Herbal Pharmacopoeia 2000:1-25. 18. Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev 2000;5:334-46. 19. Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol 1997;35:236-9. 20. Davis L, Kuttan G. Effect of Withania somnifera on cyclophosphamide-induced urotoxicity. Cancer Lett 2000;148:9-17. 21. Archana R, Namasivayam A. Antistressor effect of Withania somnifera. J Ethnopharmacol 1999;64:91-3. 22. Davis L, Kuttan G. Suppressive effect of cyclophosphamide-induced toxicity by Withania somnifera extract in mice. J Ethnopharmacol | |
Astragalus
Also Known As: Astragali Membranaceus. Scientific Name:Astragalus membranaceus. Family: Fabaceae/Leguminosae or Papilionaceae. People Use This For: Astragalus is used for common cold, upper respiratory infections, allergic rhinitis, swine ‘flu, to strengthen and regulate the immune system, fibromyalgia, anemia, and HIV/AIDS, chronic fatigue syndrome, as an antibacterial, antiviral, tonic, liver protectant, anti-inflammatory, antioxidant, as a diuretic, vasodilator, and as a hypotensive agent. Topically, astragalus is used as a vasodilator and to speed healing. Safety: No concerns regarding safety. Pregnancy and Breast Feeding: Refer to a Medical Herbalist. Effectiveness: Not enough scientific information available to comment. Allergic rhinitis: Preliminary clinical research shows that a specific astragalus root extract standardized to contain 34% polysaccharides twice daily for 3 to 6 weeks significantly improves symptoms such as runny nose, sneezing, and itching compared to placebo.1 Breast cancer: There is preliminary evidence that adjunctive use of astragalus in combination with glossy privet (Ligustrum lucidum) might increase survival rates in patients being treated conventionally for breast cancer.2 Common cold: There is preliminary evidence that long-term ingestion of astragalus might reduce the risk of catching the common cold.2 Hepatitis: There is preliminary evidence that intravenous use of astragalus might be beneficial for patients with chronic hepatitis.2 Mechanism of Action: The part used is the root. Astragalus contains a variety of active constituents including more than 34 saponins such as astragaloside, several flavonoids including isoflavones, pterocarpans, and isoflavans, polysaccharides, multiple trace minerals, amino acids, and coumarins.2,3 Astragalus has antioxidant effects. It inhibits free radical production, increases superoxide dismutase, and decreases lipid peroxidation.2,4 Astragalus is often promoted for its effects on the immune system, liver, and cardiovascular system. Astragalus seems to improve the immune response. In vitro, the polysaccharide constituents appear to bind and activate B cells and macrophages, but not T cells.5 Astragalus potentiates the effects of interferon, increases antibody levels of immunoglobulins in nasal secretions, and increases interleukin-2 levels.2,6,7 It also seems to improve the response of mononuclear cells and stimulate lymphocyte production.8 Additionally, there is preliminary evidence that astragalus extracts can restore or improve immune function in cases of immune deficiency.9,10 Astragalus seems to restore in vitro T-cell function which is suppressed in cancer patients.9,11 Astragalus also seems to have broad-spectrum in vitro antibiotic activity.2 There is interest in astragalus for increasing fertility. In vitro, astragalus appears to increase sperm motility.12 In individuals with chronic hepatitis, astragalus seems to improve liver function as demonstrated by improvement in serum glutamate pyruvate transaminase levels.2 Astragalus is also thought to cause vasodilation and increase cardiac output which might be beneficial in angina, congestive heart failure, and post-myocardial infarction.2 In animal models of heart failure, astragalus appears to increase myocardial and renal function, possibly due to diuretic and natriuretic effects.13 In animal models of coxsackie viral myocarditis, astragalus appears to reduce myocardial lesion size and viral titers.14 A pharmacokinetic evaluation in vitro and in a healthy human volunteer, suggests that astragalus flavonoids can be absorbed in the gastrointestinal tract. The major metabolites of the flavonoid constituents are glucuronides.15 These help with excretion of toxic substances. Adverse Reactions: Astragalus root is well-tolerated. Interactions with Herbs & Supplements: None known. Interactions with Drugs: Cyclophosphamide, Immunosuppressants, Lithium Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: Autoimmune Diseases: Refer to Medical Herbalist Dosage/Administration: Dr Clare’s Blends: 3ml of 1:3 tincture/day = 1gm per day. Oral: For allergic rhinitis, a specific astragalus root extract standardized to contain 34% polysaccharides (Lectranal) 160 mg twice daily has been used.1 For prevention of the common cold, 4-7 grams per day is commonly used.2 Traditionally, astragalus powder 1-30 grams per day is used.16,2 In some cases, people have used astragalus powder 30-54 grams per day.2 However, this should be avoided because some research suggests that doses greater than 28 grams per day offers no additional benefit and might even cause immune suppression.3 Astragalus decoction 0.5-1 L per day (maximum of 120 grams of whole root per liter of water) has been used.2 As a soup, mix 30 grams in 3.5 L of soup and simmer with other food ingredients.2 Topical: No typical dosage. Specific References: ASTRAGALUS Matkovic Z, Zivkovic V, Korica M, et al. Efficacy and safety of Astragalus membranaceus in 1. the treatment of patients with seasonal allergic rhinitis. Phytother Res 2010;18:175-81. 2. Upton R, ed. Astragalus Root: Analytical, quality control, and therapeutic monograph. Santa Cruz, CA: American Herbal Pharmacopoeia. 1999:1-19. 3. McCulloch M, See C, Shu XJ, et al. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: meta-analysis of randomized trials. J Clin Oncol 2006;18:419-30. Hong CY, Lo YC, Tan FC, et al. Astragalus membranaceus and Polygonum multiflorum 4. protect rat heart mitochondria against lipid peroxidation. Am J Chin Med 1994;22:57-70. 5. Shao BM, Xu W, Dai H, et al. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun 2004;320:1103-11. 6. Qun L, Luo Q, Zhang ZY, et al. Effects of astragalus on IL-2/IL-2R system in patients with maintained hemodialysis. Clin Nephrol 1999;46:333-4. 7. Hou YD, Ma GL, Wu SH, et al. Effect of Radix Astragali seu Hedysari on the interferon system. Chin Med J (Engl) 1981;94:29-34. Sun Y, Hersh EM, Lee SL, et al. Preliminary observations on the effects of the Chinese 8. medicinal herbs Astragalus membranaceus and Ligustrum lucidum on lymphocyte blastogenic responses. J Biol Response Mod 1983;2:227-31. Sun Y, Hersh EM, Talpaz M, et al. Immune restoration and/or augmentation of local graft 9. versus host reaction by traditional Chinese medicinal herbs. Cancer 1983;46:70-3. 10. Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. II. Reversal of cyclophosphamide-induced immune suppression by administration of fractionated Astragalus membranaceus in vivo. J Clin Lab Immunol 1988;19:125-9. 11. Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. I. Immune restoration of local xenogeneic graft-versus-host reaction in cancer patients by fractionated Astragalus membranaceus in vitro. J Clin Lab Immunol 1988;19:119-17. 12. Hong CY, Ku J, Wu P. Astragalus membranaceus stimulates human sperm motility in vitro. Am J Chin Med 1992;20:289-94. 13. Ma J, Peng A, Lin S. Mechanisms of the therapeutic effect of astragalus membranaceus on sodium and water retention in experimental heart failure. Chin Med J (Engl) 1998;111:17-17. 14. Yang YZ, Jin PY, Guo Q, et al. Treatment of experimental Coxsackie B-3 viral myocarditis with Astragalus membranaceus in mice. Chin Med J (Engl) 1990;103:14-8. 15. Xu F, Zhang Y, Xiao S, et al. Absorption and metabolism of Astragali radix decoction: in silico, in vitro, and a case study in vivo. Drug Metab Dispos 2006;28:913-18. 16. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. | |
B |
|---|
Berberisvulg
Scientific name: Berberis vulgaris Family: Berberidaceae. Traditiona Uses: Orally, the fruit of European barberry is used for kidney, urinary tract, and gastrointestinal tract discomforts such as heartburn, stomach cramps, constipation, lack of appetite, liver and spleen disease, for bronchial and lung discomforts, spasms, as a stimulant for circulation, for people susceptible to infection, and as a supplemental source of vitamin C. The bark, root, and root bark of European barberry are also used orally for ailments and complaints of the GI tract, liver, gallbladder, kidney and urinary tract, respiratory tract, heart and circulatory system, to lower a fever, "blood purifier", and for narcotic withdrawal. European barberry root bark is used for liver dysfunction, gallbladder disease, jaundice, splenopathy, diarrhea, indigestion, hemorrhoids, renal and urinary tract diseases, gout, rheumatism, arthritis, mid and low back pain, malaria, and leishmaniasis. Safety: Traditionally used as a fruit syrup and preserve.when the fruit is consumed orally in food amounts
Evidence from Scientific Research Very little clinical research has been done. Dental Plaque: Preliminary clinical research suggests that brushing with a European barberry extract gel containing 1% berberine three times daily for 3 weeks significantly reduces plaque index compared to placebo and has similar effects compared to a commercial antiplaque toothpaste (Colgate) Diabetes. Small studies suggests that taking European barberry for 8 weeks does not affect blood sugar control in patients with type 2 diabetes How might it work: The applicable parts of European barberry are the fruit (berry), root, bark, and root bark. Preliminary research suggests that European barberry fruit has antihistaminic and anticholinergic effects Preliminary research suggests berberine might inhibit bacterial sortase, a protein responsible for anchoring gram-positive bacteria to cell membranes. Preliminary clinical research suggests it might be useful for topical treatment of burns and trachoma, a common cause of blindness in developing countries. Unwanted effects: Orally, the use of European barberry and other berberine-containing herbs during pregnancy, lactation, or in newborn infants can cause jaundice as the infant have not yet the capacity to metabolise berberine. No adverse reactions have been reported for berberis vulgaris and no clinical studies have been done. In traditional use it is well tolerated. Berberine, a primary constituent of European barberry, has been used orally in adults in doses up to 2 grams/day for 8 weeks with no adverse effects reported Interactions with drugs/supplements: Anticoagulants/antiplatelet drugs: No reported cases of blood clotting problems have been reported with this herb. However Berberine, one of the many constituents of European barberry, may inhibit platelet aggregation in animal studies so caution is advised. Blood sugar control: Clinical study demonstrated no blood sugar lowering effect, but advise monitoring for diabetics as theoretically a lowering of blood sugar is possible. Lowering Blood Pressure: Theoreticall an effect of lowering blood pressure may add to the therapeutic effect of medication, as a precaution remain seated or lie down for 30 minutes after the first 2-3 doses and monitor effect. Transplant antirejection Cyclosporin A (CsA): The constituent Berberine can markedly elevate the blood concentration of CsA in renal-transplant recipients in both clinical and pharmacokinetic studies. This combination may allow a reduction of the CsA dosage. The mechanism for this interaction is most likely explained by inhibition of cytochrome CYP3A4 in the liver and/or small intestine. CYP3A4 Metabolised Drugs: Theoretically blood levels can be elevated or lowered. Common drugs include the SSRI antidepressants, Some Statins (lovastatin), some antibiotics, for full list see http://www.pharmacytimes.com/publications/issue/2008/2008-09/2008-09-8687 Effect on lab. tests: Theoretically, European barberry might increase bilirubin levels. This has been demonstrated with isolated berberine constituent, but not specifically with European barberry. Berberine displaces bilirubin from albumin and increases total and unbound bilirubin concentrations. Effect on conditions or disorders: BLEEDING DISORDERS: Berberine, a constituent of European barberry, might inhibit platelet aggregation. Theoretically, European barberry might increase the risk of bleeding and interfere with therapy in patients with bleeding conditions. Dose: ORAL: No typical dosage. Traditionally, a typical dose is one cup tea.To make tea, steep 1-2 teaspoons of whole or squashed berries in 150 mL boiling water 10-15 minutes and strain or steep 2 grams of root bark in 250 mL boiling water 5-10 minutes and strain. Root bark is typically used as a tincture (1:10), 20-40 drops per day. | |
Black CohoshAlso Known As: Cimicifuga, Scientific Name: Cimicifuga racemosa. Family: Ranunculaceae. People Use This For: Black Cohosh is used for symptoms of menopause, inducing labor in pregnant women, premenstrual syndrome (PMS), painful periods, nervous tension, indigestion, rheumatism, and as a mild sedative. Safety: Possibly safe when used orally and appropriately. Black cohosh has been safely used in some studies lasting up to a year; 1,2,3 There is concern that black cohosh might cause liver damage in some patients. Several case reports link black cohosh to liver failure or autoimmune hepatitis; 4,5,6,7,8,9,10,11,12,13,14,15,16,17 however, there is no conclusive evidence that black cohosh is the cause of liver damage in these patients.18 Until more is known, monitor liver function in patients who take black cohosh for more than three months. Analysis of 9 Cases of Suspected association between Black Cohosh and Hepatitis concluded that there is little if any hepatotoxic risk by the use of Black Cohosh in these cases. Menopause 2009 Sep-Oct: 16(5):956-65 Teschke R, Bahre R, Fuchs J, Wolff A. It is concluded that the use of BC may not exert an overt toxicity risk, but quality problems in a few BC products were evident that require additional regulatory quality specifications. Ann Hepatology. 2011 Jul-Sep;10(3):249-59 Pregnancy and Lactation: Refer to Medical Herbalist Effectiveness: POSSIBLY EFFECTIVE Menopausal symptoms. Some black cohosh extracts seem to modestly reduce symptoms of menopause, such as hot flashes. However, there is considerable variability in the preparations used in clinical trials, and in the results obtained.19 INSUFFICIENT RELIABLE EVIDENCE to RATE Osteoporosis: Preliminary clinical research suggests that postmenopausal women who take a specific black cohosh extract CR BNO 1055 (Klimadynon/Menofem, Bionorica AG) 40 mg/day have increased levels of bone-specific alkaline phosphatase (bALP), which is a marker of bone formation, after 12 weeks of treatment. 20 However, it is not known if this black cohosh extract can increase bone mineral density or decrease the risk of fracture. More evidence is needed to rate black cohosh for these uses. Mechanism of Action: The applicable parts of black cohosh are the rhizome and root. The active constituents of black cohosh include phytosterin; isoferulic acid; fukinolic acid; caffeic acid; salicylic acid; sugars; tannins; long-chain fatty acids; and triterpene glycosides, including acetein, cimicifugoside, and 27-deoxyactein.21,22 Black cohosh has estrogen-like effects (not estrogenic effects) that are exerted by an unknown mechanism.21,23,20 Adverse Reactions: Black cohosh can commonly cause gastrointestinal upset.4,24,25 Black cohosh has associated with weakness and muscle damage in one case. In another case, a single patient developed symptoms of cutaneous pseudolymphoma 6 months after starting a specific black cohosh extract (Remifemin). Symptoms resolved within 12 weeks of discontinuing black cohosh.26 There are two case reports of cutaneous vasculitis in menopausal women who took black cohosh-containing products. In these cases, both women were taking a combination product containing black cohosh 40 mg (Estroven, Amerifit Brands). Symptoms resolved within 3 months of discontinuing the product.27 Interactions with Herbs & Supplements: Refer to a Medical Herbalist. Interactions with Drugs: Atorvastatin (Lipitor) One report of significant interaction. Chemotherapy: Refer to a Medical Herbalist. Hepatotoxic Drugs: Refer to a Medical Herbalist. Interactions with Foods: None known. Interactions with Lab Tests: Liver Function Tests: Elevated liver function tests have not been documented in clinical trials.20 Theoretically, some patients taking black cohosh might experience elevated liver function tests. Interactions with Diseases or Conditions: Breast Cancer: Refer to Medical Herbalist. Hormone-Sensitive Cancers/Conditions: Black cohosh doesn't seem to affect estrogen receptors. Refer to Medical Herbalist. Kidney Transplant: Refer to Medical Herbalist. Liver Disease: Refer to Medical Herbalist. Dosage/Administration: Dr Clare’s Blend: 1gm/day Oral: 0.5-1gms/day Specific References: BLACK COHOSH 1. Raus K, Brucker C, Gorkow C, Wuttke W. First-time proof of endometrial safety of the special black cohosh extract (Actaea or Cimicifuga racemosa extract) CR BNO 1055. Menopause 2006;13:678-91. Newton KM, Reed SD, LaCroix AZ, et al. Treatment of vasomotor symptoms of menopause 2. with black cohosh, mulitbotanicals, soy, hormone therapy, or placebo. Ann Intern Med 2006;145:869-79. Available at: http://www.annals.org/cgi/reprint/145/12/869.pdf. 3. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause 2009;16:1156-66. 4. Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust 2002;177:440-3. 5. Lontos S, Jones RM, Angus PW, Gow PJ. Acute liver failure associated with the use of herbal preparations containing black cohosh. Med J Aust 2003;179:390-1. 6. Cohen B, Schardt D. Center for Science in the Public Interest. Letter to Food and Drug Administration. Commissioner Mark McClellan, MD, PhD. March 4, 2004. Cohen SM, O'Connor AM, Hart J, et al. Autoimmune hepatitis associated with the use of 7. black cohosh: a case study. Menopause 2004;11:575-7. 8. Levitsky J, Alli TA, Wisecarver J, Sorrell MF. Fulminant liver failure associated with the use of black cohosh. Dig Dis Sci 2005;50:538-9. 9. MHRA. Black cohosh (Cimicifuga racemosa) - risk of liver problems. Herbal Safety News IdcService=SS_GET_PAGE&useSecondary= true&ssDocName=CON2024131&ssTargetNodeId=663. 10. Lynch CR, Folkers ME, Hutson WR. Fulminant hepatic failure associated with the use of black cohosh: a case report. Liver Transpl 2006;12:989-92. 11. Chow ECY, Teo M, Ring JA, Chen JW. Liver failure associated with the use of black cohosh for menopausal symptoms. Med J Aust 2008;188:420-2. 12. Mahady GB, Low Dog T, Barrett ML, et al. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008;15:628-38. 13. Hepatotoxicity with black cohosh. Australian Adv Drug Reactions Bull 2006;25:6. Available at: www.tga.gov.au/adr/aadrb/aadr0604.htm#a1. 14. Dunbar K, Solga SF. Black cohosh, safety, and public awareness. Liver Int 2007;27:1017. 15. Patel NM, Derkits RM. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Pract 2007;20:341-6. 16. Joy D, Joy J, Duane P. Black cohosh: a cause of abnormal postmenopausal liver function tests. Climacteric 2008;11:84-8. 17. Australian Adverse Drug Reactions Advisory Committee. Black cohosh and liver toxicity - an update. Aust Adv Drug Reactions Bull 2007;26:11. 18. Teschke R, Bahre R, Genthner A, et al. Suspected black cohosh hepatotoxicity - challenges July 2006. Available at: http://www.mhra.gov.uk/home/idcplg? and pitfalls of causality assessment. Maturitas 2009;63:302-14. 19. Shams T, Setia MS, Hemmings R, et al. Efficacy of black cohosh-containing preparations on menopausal symptoms: a meta-analysis. Altern Ther Health Med 2010;16:36-44. 20. Wuttke W, Gorkow C, Seidlova-Wuttke D. Effects of black cohosh (Cimicifuga racemosa) on bone turnover, vaginal mucosa, and various blood parameters in postmenopausal women: a double-blind, placebo-controlled, and conjugated estrogens-controlled study. Menopause 2006;13:185-96. 21. Kruse SO, Lohning A, Pauli GF, et al. Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med 1999;65:763-4. 22. Loser B, Kruse SO, Melzig MF, Nahrstedt A. Inhibition of neutrophil elastase activity by cinnamic acid derivatives from Cimicifuga racemosa. Planta Med 2000;66:751-3. Einer-Jensen N, Zhao J, Andersen KP, Kristoffersen K. Cimicifuga and Melbrosia lack 23. oestrogenic effects in mice and rats. Maturitas 1996;25:149-53. 24. Pepping J. Black cohosh: Cimicifuga racemosa. Am J Health Syst Pharm 1999;56:1400-2. 25. Liske E. Therapeutic efficacy and safety of Cimicifuga racemosa for gynecologic disorders. Adv Ther 1998;15:45-53. 26. Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology 2007;214:94-6. 27. Ingraffea A, Donohue K, Wilkel C, Falanga V. Cutaneous vasculitis in two patients taking an herbal supplement containing black cohosh. J Am Acad Dermatol 2007;56:S124-6. | |
Bladderwrack
Scientific Name: Fucus vesiculosus; Ascophyllum nodosum; other Fucus species. Family: Fucaceae. People Use This For: Bladderwrack is used for thyroid disorders, iodine deficiency, goiter, obesity, arthritis, and rheumatism, "blood cleansing", to increase energy, constipation, bronchitis, decreased resistance to disease, and anxiety. Topically, bladderwrack is used for skin diseases, burns, aging skin, and insect bites. Safety: No concerns regarding safety when used orally in appropriate doses. It is important to obtain traceable supply free from contamination (1 case of contamination with heavy metals reported in the 1970’s).1,2 Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Obesity: Preliminary clinical research suggests that bladderwrack in combination with lecithin and vitamins doesn't result in sustained weight loss. More evidence is needed to rate bladderwrack for this use (combined lecithin, kelp, multivitamin preparation involving 120 women over 2 years). 3 Mechanism of Action: The applicable part of bladderwrack is the entire plant. Bladderwrack is a brown seaweed. Bladderwrack contains high concentrations of iodine, which is present in varying amounts. Bladderwrack is a source of fiber, minerals such as iron, and vitamin B12.2 Preliminary clinical research suggests bladderwrack may normalize the menstrual cycle and have estrogen balancing effects in premenopausal women. It may also balance progesterone effects4 (case reports on three patients). Preliminary clinical research suggests topical administration of bladderwrack extract might reduce skin thickness and other signs of aging.5 Adverse Reactions: Excess Iodine intake is rare in humans outside of radiation contamination or excessive amounts of seaweed (or seaweed extracts) intake over a prolonged period. There is one case report of heavy metal poisoning where arsenic poisoning occurred with ingestions of a contaminated kelp product. 6 Another case of arsenic-related poisoning with bladderwrack ingestion 400 mg three times a day for 3 months resulted in kidney damage.7 Interactions with Herbs & Supplements: Avoid Iodine supplements at the same time. Interactions with Drugs: Antithyroid Drugs: Theoretically, may result in additive hypothyroid activity, and may lower the level of availableThyroid hormones.8 Interactions with Foods: None known. Interactions with Lab Tests: Radioactive Iodine Uptake: Theoretically, bladderwrack might interfere with the results of thyroid function tests using radioactive iodine uptake.2 Thyroid Stimulating Hormone (TSH): Theoretically, bladderwrack might increase serum TSH levels and test results.8 Thyroxine (T4): Theoretically, bladderwrack might increase serum T4 levels and test results.8 Interactions with Diseases or Conditions: Iodine Allergy: Avoid bladderwrack use in people sensitive to iodine.8 Thyroid Disorders: Prolonged use or excessive amounts of iodides may exacerbate thyroid gland problems.8 Dosage/Administration: Dr Clare’s Blends: 1 gm per day No typical dosage. Specific References: BLADDERWRACK 1. Baker DH. Iodine toxicity and its amelioration. Exp Biol Med (Maywood) 2004;229:473-8. 2. Phaneuf D, Cote I, Dumas P, et al. Evaluation of the contamination of marine algae (Seaweed) from the St. Lawrence River and likely to be consumed by humans. Environ Res 1999;80:S175-S182. 3. Bjorvell H, Rössner S. Long-term effects of commonly available weight reducing programmes in Sweden. Int J Obes 1987;11:67-71. 4. Skibola CF. The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: a case report. BMC Complement Altern Med 2004;4:10. 5. Fujimura T, Tsukahara K, Moriwaki S, et al. Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. J Cosmet Sci 2002;53:1- Pye KG, Kelsey SM, House IM, et al. Severe dyserythropoeisis and autoimmune 6. thrombocytopenia associated with ingestion of kelp supplement. Lancet 1992;339:1540. 7. Conz PA, La Greca G, Benedetti P, et al. Fucus vesiculosus: a nephrotoxic alga? Nephrol Dial Transplant 1998;13:526-7. 8. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2002. Available at: www.nap.edu/books/0309072794/html/. | |
Bogbean
Buckbean, Marsh Trefoil, Menyanthes, Water Shamrock. Scientific Name: Menyanthes trifoliata. Family: Menyanthaceae. People Use This For: Bogbean is used for rheumatism, rheumatoid arthritis, loss of appetite, and dyspepsia. In food manufacturing, bogbean is used as a flavoring agent. Safety: No concerns regarding safety when used orally in amounts commonly found in foods.1 No concerns regarding safety when used orally in medicinal amounts,2 no clinical reports of problems. Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available to comment. Mechanism of Action: The applicable part of bogbean is the leaf. The bitter principles, or iridoids, can stimulate saliva and gastric juices (3,1). Bogbean can have purgative actions (1). Adverse Reactions: None reported for normal dosage. Interactions with Herbs & Supplements: None reported Interactions with Drugs: None reported. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None reported. Dosage/Administration: Dr Clare’s Blends:1gm per day Oral: The typical dose of bogbean is 1-3 grams of the dried leaf three times daily or as a tea three times daily. Specific References: BOGBEAN Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare 1. Professionals. London, UK: The Pharmaceutical Press, 1996. 2. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 3. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. | |
Boneset
Agueweed, Crosswort, Eupatoire, Eupatorio, Feverwort, Indian Sage, Sweating Scientific Name: Eupatorium perfoliatum. Family: Asteraceae/Compositae. People Use This For: Orally, boneset is used as an antipyretic, diuretic, laxative, emesis, and cathartic. Safety: POSSIBLY UNSAFE: When used orally in excessive amounts. Large doses are both cathartic and emetic. Though the alkaloids have not been characterized, hepatotoxic pyrrolizidine alkaloids (PAs) are common in this genus (3). PREGNANCY AND LACTATION: POSSIBLY UNSAFE ...when used orally, due to possible hepatotoxic pyrrolizidine alkaloid content (3); avoid using. Effectiveness: There is insufficient reliable information available about the effectiveness of boneset. Mechanism of Action: The applicable parts of boneset are the dried leaf and flowering parts. Preliminary research suggests boneset might have cytotoxic and mild antibacterial activity (4). Adverse Reactions: Report an Adverse Reaction to BONESET Orally, boneset can cause an allergic reaction in individuals sensitive to the Asteraceae / Compositae family. Members of this family include ragweed, chrysanthemums, marigolds, daisies, and many other herbs. Interactions with Herbs & Supplements: HEPATOTOXIC : Concomitant use is contraindicated due to the risk of additive toxicity. Herbs containing hepatotoxic PAs include borage, butterbur, coltsfoot, comfrey, gravel root, hemp agrimony, hound's tongue, and the Senecio species plants dusty miller, alpine ragwort, groundsel, golden ragwort, and tansy ragwort (2). PYRROLIZIDINE ALKALOID (PA)-CONTAINING Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: CROSS-ALLERGENICITY: Boneset can cause an allergic reaction in individuals sensitive to the Asteraceae/Compositae family. Members of this family include ragweed, chrysanthemums, marigolds, daisies, and many other herbs. Dosage/Administration: ORAL: Traditionally one cup of tea, prepared by steeping 1-2 grams herb in 150 mL boiling water, has been used three times daily. The liquid extract, 1:1 in 25% alcohol, has been used 1-2 mL three times daily. 1-4 mL of the tincture, 1:5 in 45% alcohol, has also been used three times daily (1). Specific References: THYME 1. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare 2. Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine 3. Roeder E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 4. Habtemariam S, Macpherson AM. Cytotoxicity and antibacterial activity of ethanol extract from leaves of a herbal drug, boneset (Eupatorium perfoliatum). Phytother Res 2000;14:575-7. | |
Boswellia
Also known as: Indian Frankincense, Indian Olibanum, Ru Xiang, Salai Guggul. Scientific name: Boswellia serrata. Botanical Family: Burseraceae. Part used: The medicinal part of Indian frankincense plant is the gum resin. The gum resin is obtained by pulling away the bark of the Indian frankincense tree. Traditional use. Indian frankincense is used for osteoarthritis, rheumatoid arthritis, rheumatism, bursitis, and tendonitis. Other uses include ulcerative colitis, abdominal pain, asthma, allergic rhinitis, sore throat, painful menstruation, acne, and cancer. It is also used as a stimulant, respiratory antiseptic, diuretic, and for stimulating menstrual flow. Safety. There are no safety concerns when used orally and appropriately. Indian frankincense has been safely used in several clinical trials lasting up to ninety days. (1,2, 3, 4, 5, 6, 7, 8, 9, 10) Pregnancy: Safe when used orally in amounts commonly found in foods.(11) For medicinal use consult an Herbal Medicine Physician. Breastfeeding: Safe when used orally in amounts commonly found in foods.(11) For medicinal use consult an Herbal Medicine Physician. Constituents Boswellic acids (triterpenoids) Essential oils including cymene and thujone Scientific evidence. Osteoarthritis. Some clinical research shows that taking specific Indian frankincense extracts can reduce symptoms of osteoarthritis. In two clinical trials, using a specific Indian frankincense extract (5-Loxin) 100 mg daily or 250 mg daily significantly improved pain and functionality scores in patients with osteoarthritis after 90 days of treatment. Pain scores were reduced by about 32% to 65%. Patients began to have significant improvement within 7 days of treatment. The extract used in this study was standardized and enriched to contain 30% of the boswellic acid AKBA. (8,9) One clinical trial evaluated another specific Indian frankincense extract (Aflapin) 100 mg daily. This extract significantly improved pain and functionality scores in patients with osteoarthritis after 90 days of treatment. Pain scores were reduced by about 47%. Patients began to have significant improvement within 7 days of treatment. The extract used in this study was standardized and enriched to contain 20% of the boswellic acid AKBA. (9) In a preliminary crossover trial, taking a different Indian frankincense extract, 333mg daily also significantly reduced symptoms of osteoarthritis, such as knee pain and swelling. (3) Ulcerative colitis. Two clinical trials show that taking Indian frankincense can improve some symptoms of ulcerative colitis and some pathological measures. In one study, taking whole plant Indian frankincense resin 350 mg three times daily significantly improved symptoms and disease markers in patients with ulcerative colitis. In this study, about 82% of patients taking Indian frankincense went into remission compared to 75% taking the commonly prescribed drug sulfasalazine.(2) In another preliminary clinical study, taking whole plant Indian frankincense resin 300 mg three times for 6 weeks improved symptoms and some measures of disease pathology in about 90% of patients. In this study 70% of patients taking Indian frankincense went into remission compared to 40% taking sulfasalazine 3 grams daily. (7) Asthma. There is some preliminary evidence that taking Indian frankincense extract orally might help asthma. It may improve forced expiratory volume, reduce the number of asthma attacks, and decrease clinical signs of asthma. (1) Crohn's disease. There is preliminary evidence that taking Indian frankincense extract orally might reduce some symptoms of Crohn’s disease. One clinical study found that it worked as well as mesalamine (Asacol, Pentasa) for Crohn's disease;(6) however, other clinical research shows that taking Indian frankincense 800 mg orally three times a day did not increase rates of remissions and quality of life any more than placebo in patients with Crohn's disease. (12) Rheumatoid arthritis. There is conflicting research about the usefulness of Indian frankincense extract taken orally for rheumatoid arthritis. (4,5) Mechanism of action. The principle constituents of Indian frankincense are boswellic acid and alpha- and beta-boswellic acid, which are thought to have anti-inflammatory properties. (13,14) The boswellic acid (AKBA) constituent appears to be the most potent anti-inflammatory constituent. (14) The gum resin also contains up to 16% essential oils including alpha-thujene and p-cymene. (15) In preliminary research, some Indian frankincense extracts show anti-inflammatory, analgesic, and anti-arthritis effects; however, not all Indian frankincense-containing products seem to have these effects. (3) Boswellic acids, especially AKBA, inhibit 5-LOX enzymes and reduce leukotriene synthesis and inhibit leukocyte elastase, which are the likely mechanisms for its anti-inflammatory properties. Boswellic acids may also have a disease modifying effect by decreasing the breakdown of glycosaminoglycan and thus reducing cartilage damage. Indian frankincense may also inhibit mediators of autoimmune disorders. It seems to reduce production of antibodies and cell-mediated immunity. (3,7,16,17) Indian frankincense may be useful in treating cancer. Preliminary research suggests that boswellic acids have an anti-proliferative effect and an effect of increasing the rate of cell death (apoptosis) in cancer cells. (16) Preliminary research suggests that boswellic acids stabilize mast cells, this suggests usefulness for asthma and other allergic conditions.(18) Other preliminary research suggests that boswellic acids might help prevent organ rejection and ischemia/reperfusion injury.(19) Indian frankincense has an elimination half-life of 6 hours from the blood stream.(20) Adverse reactions. Indian frankincense is well-tolerated. Side effects reported in clinical trials did not occur more commonly than placebo. (8) Some reported side effects include diarrhea, nausea, abdominal pain, and heartburn. (3,7,8 ,9 ,10) No serious adverse events have been documented.(10) Interactions with herbs and supplements. None known. Interactions with drugs. None known. Interactions with foods. None known. Interactions with lab tests. None known. Interactions with diseases or conditions. None known. Dosage. For osteoarthritis, a specific Indian frankincense extract (5-Loxin) 100 mg daily or 250 mg daily has been used.(8) Indian frankincense extract 333 mg three times daily has been used. (3) For rheumatoid arthritis, Indian frankincense extract 3,600 mg daily has been used. (4) For Crohn's disease, 800 mg three times daily has been used. (21) For ulcerative colitis, a gum resin preparation of 300-350 mg three times daily has been used. (2,7) For asthma, 300 mg three times daily has been used. (1) | |
Buchu
Barosmae Folium. Scientific Name: Barosma betulina People Use This For: Orally, buchu is used as a urinary tract disinfectant in cystitis, urethritis, prostatitis, benign prostatic hyperplasia and kidney infections. In manufacturing, the oil from buchu is used to give a fruit flavor (often black currant) to foods. Safety: No concerns regarding safety, available studies validate this statement, when the leaf is used in amounts commonly found in foods. Buchu has Generally Recognized As Safe status (GRAS) for use in foods in the US.1 No converns regarding safety when the leaf is used orally and appropriately in medicinal amounts.2,3 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: Not enough scientific information gathered to offer a comment Mechanism of Action: The applicable part of buchu is the leaf. Buchu camphor (also known as diosphenol) is the principal constituent of the oil. Researchers believe this constituent may be responsible for buchu's reported diuretic and antiseptic effects.4 Adverse Reactions: Occasional digestive upset if taken on an empty stomach.R1 pp.311 Interactions with Herbs & Supplements: None Interactions with Drugs: Lithium: Because of diuretic effect.R3 pp.163-164 Diuretics: Effect can be additive.R4 pp.192,204,215 Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: Surgery: Tell patients to discontinue buchu at least 2 weeks before elective surgical procedures. Dosage/Administration: Oral: The typical dose is 1 cup of tea (steep 1 gram dry leaf in 150 mL boiling water 5-10 minutes, strain) several times per day.5 Specific References: BUCHU 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 2. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 3. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 4. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 5. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. Stuttgart: Medpharm GmbH Scientific Publishers, 1994. | |
Burdock Root
Arctium, Beggar's Buttons, Burr Seed, Clotbur, Cocklebur. Scientific Name: Arctium lappa; Arctium minus; Arctium tomentosum. Family: Asteraceae/Compositae. People Use This For: Burdock is used as a diuretic, "blood purifier", antimicrobial, and an antipyretic. It is also used to treat gastrointestinal complaints, rheumatism, gout, cystitis, and chronic skin conditions including acne and psoriasis. It is also used for hypertension, arteriosclerosis, hepatitis, and other inflammatory conditions. SourceURL:file://localhost/Users/ruthruane/Downloads/Herbal%20Medicine-Newest.doc Burdock is also used for treating colds, catarrh. Topically, burdock is used for dry skin, acne, psoriasis, and eczema. The root of burdock is consumed as a food. Safety: No concerns regarding safety when used in amounts commonly found in foods.9,10 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available to comment on the effectiveness of burdock. Mechanism of Action: The applicable relevant part of burdock is the root. Extracts of burdock root appear to have cough suppressant activity and may increase immunological activity.11 Other preliminary research suggests it might have anti-inflammatory and free radical scavenging activity.12 Burdock root extract might also protect the liver from toxicity caused by ethanol and carbon tetrachloride, possibly due to its antioxidant activity.9 Adverse Reactions: An isolated report of an allergic reaction causing anaphylaxis.10 Interactions with Foods: None known. Interactions with Lab Tests: SourceURL:file://localhost/Users/ruthruane/Downloads/Herbal%20Medicine-Newest.doc None known. Dosage/Administration: No typical dosage Specific References: BURDOCK 9. Lin SC, Lin CH, Lin CC, et al. Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J Biomed Sci 2002;9:401-9. 10. Sasaki Y, Kimura Y, Tsunoda T, Tagami H. Anaphylaxis due to burdock. Int J Dermatol 2003;42:472-3. 11. Kardosova A, Ebringerova A, Alfoldi J, et al. A biologically active fructan from the roots of Arctium lappa L., var. Herkules. Int J Biol Macromol 2003;33:135-40. 12. Lin CC, Lu JM, Yang JJ, et al. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am J Chin Med 1996;24:127-37. | |
C |
|---|
Catmint
Cataire, Catmint, Catswort, Chataire, Field Balm, Hierba Gatera, Menta de Gato, Menthe des Chats. CAUTION: See separate listing for Schizonepeta. Scientific Name: Nepeta cataria. Family: Lamiaceae/Labiatae. People Use This For: Catnip is used for insomnia; migraine headaches; cold; flu; swine flu; fever; hives; and gastrointestinal (GI) upset, including indigestion, colic, cramping, and flatulence. It is also used orally for conditions associated with anxiety, diuresis, as a tonic, for upper respiratory tract infections, and headaches. Additionally, catnip is also used orally for lung and uterine congestion, eradicating worms, and for initiating menses in girls with delayed onset of menstruation. Topically, catnip has been used for arthritis, hemorrhoids, and as a poultice to relieve swelling. As an inhalant, catnip is smoked for respiratory conditions and recreationally for inducing a euphoric high. In manufacturing, catnip is used as a pesticide and insecticide. Safety: POSSIBLY SAFE ...when used orally and appropriately (2,3). Significant adverse effects have not been reported when catnip tea is used in cupful amounts (62). POSSIBLY UNSAFE : when used orally in excessive doses. Higher doses may be associated with significant adverse effects (62). ...when inhaled by smoking dried leaves. Smoking the dried leaves of catnip has been associated with a euphoric high (2), which might impair judgment; however, whether catnip can truly produce this effect in humans remains controversial (2). There is insufficient reliable information available about the safety of topically applied catnip. CHILDREN: POSSIBLY UNSAFE ...when used orally. One child developed stomach pain and irritability followed by lethargy and hypnotic state after ingesting catnip leaves and tea (1,5). PREGNANCY: LIKELY UNSAFE ...when used orally. Catnip tea has been reported to have uterine stimulant properties (3); avoid using. LACTATION: Insufficient reliable information available; avoid using. Effectiveness: There is insufficient reliable information available about the effectiveness of catnip. Mechanism of Action: The applicable part of catnip is the flowering tops. The pharmacological effect that catnip is famous for is the euphoric state it induces in cats. It is thought that the constituent cis-trans-nepetalcatone produces the characteristic stimulation in cats only when they smell it (1). Although humans have used catnip to induce a euphoric high, whether or not this effect actually occurs in humans is controversial. In humans, the constituent nepetalactone is thought to be responsible for catnip's calming effects in insomnia, anxiety, gastrointestinal (GI) conditions, and migraine headache. Nepetalactone is the major component (80- 95%) of the volatile oil of catnip and is structurally related to the valepotriates found in valerian. Catnip provides approximately 0.2-1% volatile oil. Catnip reportedly also has antipyretic and diaphoretic effects, which have been attributed to its use for colds, flu, and fever. Other reported pharmacological effects, include diuretic and stimulation of gallbladder activity (2). Adverse Reactions: Report an Adverse Reaction to CATNIP Catnip abuse may result in headache and malaise. Large amounts of tea may cause vomiting (2). One case report exists of a nineteen-month-old child who developed a stomachache and irritability, followed by lethargy and a hypnotic state after ingesting raisins soaked in catnip tea and chewing on the tea bag (5). Interactions with Herbs & Supplements: HERBS AND SUPPLEMENTS WITH SEDATIVE PROPERTIES: Theoretically, concomitant use of catnip with herbs that have sedative properties might enhance therapeutic and adverse effects. Some of these supplements include 5-HTP, calamus, California poppy, catnip, hops, Jamaican dogwood, kava, St. John's wort, skullcap, valerian, yerba mansa, and others. Interactions with Drugs: CNS DEPRESSANTS <<interacts with>> CATNIP Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = D Theoretically, concomitant use with drugs with sedative properties may cause additive effects and side effects (4). LITHIUM <<interacts with>> CATNIP Interaction Rating = Moderate Be cautious with this combination. Catnip is thought to have diuretic properties. Theoretically, due to these potential Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: PELVIC INFLAMMATORY DISEASE (PID) and MENORRHAGIA: Because catnip is also used to stimulate menstruation, theoretically it is contraindicated in pelvic inflammatory disease (PID) and excessive menstrual bleeding (3). SURGERY: Catnip has CNS depressant effects. Theoretically, catnip might cause additive CNS depression when combined with anesthesia and other medications during and after surgical procedures. Tell patients to discontinue catnip at least 2 weeks before elective surgical procedures. Dosage/Administration: ORAL: People typically use two 380 mg capsules three times daily at meals or prepared as a tea using 1-2 teaspoons in 6 ounces of boiling water. Specific References: Catmint 1. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 2. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 3. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 4. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. 5. Osterhoudt KC, Lee SK, Callahan JM, Henretig FM. Catnip and the alteration of human consciousness. Vet Hum Toxicol 1997;39:373-5. | |
Cayenne
African Bird Pepper, African Chillies, African Pepper, Aji, Bird Pepper, Capsaicin, Capsaïcine, Cayenne, Cayenne Pepper, Chili, Chili Pepper, Chilli, Chillies, Cis-capsaicin, Civamide, Garden Pepper, Goat's Pod, Grains of Paradise, Green Chili Pepper, Green Pepper, Hot Pepper, Hungarian Pepper, Ici Fructus, Katuvira, Lal Mirchi, Louisiana Long Pepper, Louisiana Sport Pepper, Mexican Chilies, Mirchi, Oleoresin Capsicum, Paprika, Paprika de Hongrie, Pili-pili, Piment de Cayenne, Piment Enragé, Piment Fort, Piment-oiseau, Pimento, Poivre de Cayenne, Poivre de Zanzibar, Poivre Rouge, Red Pepper, Sweet Pepper, Tabasco Pepper, Trans-capsaicin, Zanzibar Pepper, Zucapsaicin, Zucapsaïcine. CAUTION: See separate listings for Grains of Paradise and Indian Long Pepper.Scientific Name:Capsicum frutescens; Capsicum annuum; Capsicum chinense; Capsicum baccatum; Capsicum pubescens; Capsicum minimum; and other Capsicum species. People Use This For: Orally, capsicum is used for dyspepsia, flatulence, colic, diarrhea, cramps, toothache, poor circulation, excessive blood clotting, seasickness, swallowing dysfunction, alcoholism, malaria, fever, hyperlipidemia, and preventing heart disease. Intranasally, capsicum is used for allergic rhinitis, perennial rhinitis, migraine headache, cluster headache, sinonasal polyposis, and sinusitis Safety: LIKELY SAFE ...when used orally in amounts typically found in food. Capsicum has Generally Recognized As Safe (GRAS) status in the US (5). ...when used topically and appropriately. The active capsicum constituent capsaicin used in topical preparations is an FDA-approved over-the-counter product (1). Effectiveness: Pain. Several clinical studies show that applying 0.25% to 0.75% capsaicin cream topically temporarily relieves chronic pain from rheumatoid arthritis, osteoarthritis, psoriasis, and neuralgias including shingles and diabetic neuropathy (13, 47). The active capsicum constituent capsaicin, used in topical preparations, is FDA-approved for these uses (1). Back pain. Some evidence shows that applying a capsicum-containing plaster to back can significantly reduce low-back pain compared to placebo (44, 45. 46). Prurigo nodularis. Applying a cream containing 0.025% to 0.3% of the active capsicum constituent capsaicin 4-6 times daily seems to relieve burning sensations, erythema, pruritus, and healing of skin lesions over a period of 2 weeks to 33 months. Symptoms, however, may return after discontinuation of therapy (48). HIV-associated peripheral neuropathy. Applying capsaicin topically does not seem to relieve symptoms of HIV-associated peripheral neuropathy (4). INSUFFICIENT RELIABLE EVIDENCE to RATE Allergic rhinitis. Preliminary research suggests that intranasal treatment with cotton wads soaked in capsaicin applied for 15 minutes and repeated over two days might reduce the severity of experimentally induced allergic rhinitis. Severity of symptoms seems to be decreased for up to two months after capsaicin treatment (30). However, contradictory evidence suggests that patients with allergic rhinitis caused by house dust mites do not have significantly improved symptoms after using a capsaicin solution providing 0.15 mg/dose for up to 7 doses over a 14-day period (42). Swallowing dysfunction. Preliminary evidence suggests that elderly patients at risk for aspiration pneumonia due to swallowing dysfunction have improved swallowing reflexes after dissolving a capsaicin-containing lozenge in their mouth before each meal (27).
Mechanism of Action:
The applicable part of capsicum is the fruit. Capsicum contains the constituent capsaicin, which makes it taste hot. Capsicum powder has been reported to prevent radiation-induced damage to bacterial DNA and thereby protect certain bacteria (Escherichia coli, Bacillus megaterium, and Bacillus pumilus spores) from gamma irradiation, which is used to preserve some foods (6).
Adverse Reactions: Orally, capsicum can cause upper abdominal discomfort including fullness, gas, bloating, nausea, epigastric pain and burning, diarrhea, and belching (12, 22). Sweating and flushing of the head and neck, lacrimation, headache, faintness, and rhinorrhea have also been reported (8, 22). Excessive amounts of capsaicin can lead to gastroenteritis and hepatic necrosis (13). There are also reports of dermatitis in breast-fed infants whose mothers' food is heavily spiced with capsicum (2). Capsicum can also decrease blood coagulation (9). Capsicum oleoresin, an oily extract in pepper self-defense sprays, causes intense eye pain. It can also cause erythema, blepharospasm, tearing, shortness of breath, and blurred vision. In rare cases, corneal abrasions have occurred (20, 21).
Interactions with Herbs & Supplements: ANTICOAGULANT/ANTIPLATELET HERBS AND SUPPLEMENTS: Concomitant use of herbs and supplements that affect platelet aggregation could theoretically increase the risk of bleeding in some people. Some of these herbs include angelica, clove, danshen, garlic, ginger, ginkgo, Panax ginseng, and others. Interactions with Drugs:
ACE INHIBITORS (ACEIs) <<interacts with>> CAPSICUM Interaction Rating = Minor Be watchful with this combination. Severity = Mild • Occurrence = Unlikely • Level of Evidence = D
There is one case report of a topically applied cream containing capsaicin contributing to the cough reflex in a patient using an ACE-inhibitor (26). (12414). But it is unclear if this interaction is clinically significant.
ANTICOAGULANT/ANTIPLATELET DRUGS <<interacts with>> CAPSICUM Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = D
Theoretically, capsicum might increase the effects and adverse effects of antiplatelet drugs (15, 16). COCAINE <<interacts with>> CAPSICUM Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = D Theoretically, concomitant use of capsicum (including exposure to the capsicum in pepper spray) and cocaine might increase cocaine effects and the risk of adverse effects, including death (3). THEOPHYLLINE <<interacts with>> CAPSICUMInteraction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Possible • Level of Evidence = D
Theoretically, oral administration of capsicum before or at the same time as theophylline might enhance theophylline absorption (14). Interactions with Foods:None known.Interactions with Lab Tests:BLEEDING TIME: Capsicum has led to increased fibrinolytic activity and may lead to prolonged times in coagulation studies (9).Interactions with Diseases or Conditions:DAMAGED SKIN: Capsicum is contraindicated in situations involving injured skin. Do not apply capsicum if the skin is open. | |
Chamomile
Blue Chamomile, Camomilla, Camomille, Camomille Allemande, Chamomilla, Echte Kamille, Feldkamille, Fleur de Camomile, Hungarian Chamomile, Kamillen, Kleine Kamille, Manzanilla, Manzanilla Alemana, Matricaire, Matricariae Flos, Pin Heads, Sweet False Chamomile, True Chamomile, Wild Chamomile. Scientific Name: Matricaria recutita, synonyms Chamomilla recutita, Matricaria chamomilla. Family: Asteraceae/Compositae. People Use This For: Orally, German chamomile is used for flatulence, travel sickness, nasal mucous membrane inflammation, allergic rhinitis, nervous diarrhea, attention deficit hyperactivity disorder (ADHD), fibromyalgia, restlessness, and insomnia. It is also used for gastrointestinal (GI) spasms, colic, inflammatory diseases of the GI tract, GI ulcers associated with nonsteroidal anti-inflammatory drugs (NSAIDs) and alcohol consumption, and as an antispasmodic for menstrual cramps. Topically, German chamomile is used for hemorrhoids; mastitis; leg ulcers; skin, anogenital, and mucous membrane inflammation; and bacterial skin diseases, including those of the mouth and gums. It is also used topically for treating or preventing chemotherapy- or radiation-induced oral mucositis. As an inhalant, German chamomile is used to treat inflammation and irritation of the respiratory tract. In foods and beverages, German chamomile is used as flavor components. In manufacturing, German chamomile is used in cosmetics, soaps, and mouthwashes. Safety: No concerns regarding safety, available studies validate this statement, when used orally in amounts commonly found in foods. German chamomile has Generally Recognized as Safe (GRAS) status in the US.1 No concerns regarding safetywhen used orally, short-term. There is some evidence that German chamomile can be used safely for up to 8 weeks.2,3,4 The long-term safety of German chamomile in medicinal doses is unknown, when used topically; avoid applying it near the eyes.5 Children: No concerns regarding safety when used orally and appropriately, short-term. Preliminary clinical research also suggests that a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German SourceURL:file://localhost/Users/ruthruane/Downloads/Herbal%20Medicine-Newest.doc chamomile 178 mg (ColiMil, Milte Italia SPA) is safe in infants when used for up to a week.6 Pregnancy and Lactation: Insufficient reliable information available; avoid using.
Effectiveness: POSSIBLY EFFECTIVE Colic. A clinical trial shows that breast-fed infants with colic who are given a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (ColiMil, Milte Italia SPA) twice daily for a week have reduced crying times compared to placebo.6
Dyspepsia. A specific combination product containing German chamomile (Iberogast, Medical Futures, Inc) seems to improve symptoms of dyspepsia. The combination includes German chamomile plus peppermint leaf, clown's mustard plant, caraway, licorice, milk thistle, celandine, angelica, and lemon balm.7,3 A meta-analysis of studies using this combination product suggests that taking 1 mL orally three times daily over a period of 4 weeks significantly reduces severity of acid reflux, epigastric pain, cramping, nausea, and vomiting compared to placebo.8
Oral mucositis. Using a German chamomile oral rinse (Kamillosan Liquidum) might help prevent or treat mucositis induced by radiation therapy and some types of chemotherapy.2 German chamomile oral rinse seems to prevent or treat mucositis secondary to radiation therapy and some types of chemotherapy including asparaginase (Elspar), cisplatin (CDDP, Platinol-AQ), cyclophosphamide (Cytoxan, Neosar), daunorubicin (DaunoXome), doxorubicin (Adriamycin, Rubex), etoposide (VP-16, Etopophos, VePesid, Toposar), hydroxyurea (Hydrea), mercaptopurine (6-MP, Purinethol), methotrexate (MTX, Rheumatrex), procarbazine (MIH, Mutlane), and vincristine (VCR, Oncovin, Vincasar) (2). However, the rinse doesn't seem to be better than placebo for preventing fluorouracil (5-FU)-induced oral mucositis.9
POSSIBLY INEFFECTIVE Dermatitis. Applying German chamomile cream topically does not seem to prevent dermatitis induced by cancer radiation therapy.10
Mechanism of Action: The applicable part of German chamomile is the flowerhead. Active constituents of German chamomile include quercetin, apigenin, and coumarins, and the essential oils.5
German chamomile might have anti-inflammatory effects. Preliminary research suggests it can inhibit the pro inflammatory enzymes. Other constituents may inhibit histamine related to allergies,5,4 The constituent(s) responsible for the sedative activity of German chamomile are unclear. Preliminary research suggests that extracts of German chamomile might inhibit morphine dependence and withdrawal.11 Other preliminary research suggests that German chamomile flower extract taken orally might have an antipruritic effect.12 Preliminary research suggests that German chamomile blocks slow wave activity in the small intestine, which could slow peristaltic movement.13
Adverse Reactions: Orally, German chamomile tea can cause allergic reactions including severe reactions in some patients.14
Interactions with Herbs & Supplements: HERBS AND SUPPLEMENTS WITH SEDATIVE PROPERTIES: Theoretically, concomitant use with herbs that have sedative properties might have additive effects which needs to be taken into account.5,16 Interactions with Drugs: Benzodiazepines: Consult a Medical Herbalist CNS Depressants: Consult a Medical Herbalist Warfarin (Coumadin): Consult a Medical Herbalist Interactions with Foods: None known. Interactions with Lab Tests: Creatinine: Chronic ingestion of German chamomile for two 2 weeks can reduce urinary creatinine output. This effect may be prolonged for up to two weeks after discontinuing German chamomile. The mechanism for this effect is unclear.4 Interactions with Diseases or Conditions: Surgery: Avoid from 2 weeks prior to elective surgery. Dosage/Administration: Oral: For dyspepsia, a specific combination product containing German chamomile (Iberogast, Medical Futures, Inc) and several other herbs has been used in a dose of 1 mL three times daily.7,3,8 For colic in infants, a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (ColiMil, Milte Italia SPA) twice daily for a week has been used.6 Topical: For chemotherapy- or radiation-induced oral mucositis, an oral rinse made with 10-15 drops of German chamomile liquid extract in 100 mL warm water has been used three times daily.2 Comments: German chamomile is an annual herb found throughout Europe and in portions of Asia. German chamomile has a mild apple-like scent. The name "chamomile" is Greek for "Earth apple." Specific References: CHAMOMILE 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 2. Carl W, Emrich LS. Management of oral mucositis during local radiation and systemic chemotherapy: a study of 98 patients. J Prosthet Dent 1991;66:361-9. 3. Madisch A, Holtmann G, Mayr G, et al. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004;69:45-52. 4. Wang Y, Tang H, Nicholson JK, et al. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J Agric Food Chem 2005;53:191-6. 5. Hormann HP, Korting HC. Evidence for the efficacy and safety of topical herbal drugs in dermatology: part I: anti-inflammatory agents. Phytomedicine 1994;1:161-71. 6. Savino F, Cresi F, Castagno E, et al. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytother Res 2005;19:335-40. 7. Holtmann G, Madisch A, Juergen H, et al. A double-blind, randomized, placebo-controlled trial on the effects of an herbal preparation in patients with functional dyspepsia [Abstract]. Ann Mtg Digestive Disease Week 1999 May. 8. Melzer J, Rosch W, Reichling J, et al. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther 2004;20:1279-87. 9. Fidler P, Loprinzi CL, O'Fallon JR, et al. Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer 1996;77:522-5. 10. Maiche AG, Grohn P, Maki-Hokkonen H. Effect of chamomile cream and almond ointment on acute radiation skin reaction. Acta Oncol 1991;30:395-6. 11. Gomaa A, Hashem T, Mohamed M, Ashry E. Matricaria chamomilla extract inhibits both development of morphine dependence and expression of abstinence syndrome in rats. J Pharmacol Sci 2003;92:50-5. 12. Kobayashi Y, Nakano Y, Inayama K, et al. Dietary intake of the flower extracts of German chamomile (Matricaria recutita L.) inhibited compound 48/80-induced itch-scratch responses in mice. Phytomedicine 2003;10:657-64. 13. Storr M, Sibaev A, Weiser D, et al. Herbal extracts modulate the amplitude and frequency of slow waves in circular smooth muscle of mouse small intestine. Digestion 2004;70:257-64. 14. Subiza J, Subiza JL, Hinojosa M, et al. Anaphylactic reaction after the ingestion of chamomile tea; a study of cross-reactivity with other composite pollens. J Allergy Clin Immunol 1989;84:353-8. 15. Viola H, Wasowski C, Levi de Stein M, et al. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med 1995;61:213-6. 16. Avallone R, Zanoli P, Puia G, et al. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol 2000;59:1387-94. | |
Cinnamon
Also Known As: Cassia Cinnamon, Canela de Cassia, Canela Molida, Canelle, Cannelle Scientific Name: Cinnamomum aromaticum, synonyms Cinnamomum cassia, and Cinnamomum People Use This For: Orally, cassia cinnamon is used for type 2 diabetes, gas (flatulence), muscle and Safety: LIKELY SAFE ...when used orally and appropriately. Cassia cinnamon has been Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Diabetes. There is contradictory evidence about the effectiveness of cassia Mechanism of Action: The applicable parts of cassia cinnamon are the bark and flower. Adverse Reactions: Orally, cassia cinnamon appears to be well-tolerated. No significant side effects Interactions with Herbs & Supplements: HEPATOTOXIC HERBS AND SUPPLEMENTS: There is some concern that Interactions with Drugs: ANTIDIABETES DRUGS <<interacts with>> CASSIA CINNAMON Interaction Rating = Moderate Be cautious with this combination. Cassia cinnamon may lower blood glucose levels, and have additive effects in HEPATOTOXIC DRUGS <<interacts with>> CASSIA CINNAMON Interaction Rating = Moderate Be cautious with this combination. Interactions with Foods: None known. Interactions with Lab Tests: BLOOD GLUCOSE: Cassia cinnamon might lower blood glucose levels and test Interactions with Diseases or Conditions: DIABETES: Cassia cinnamon might lower blood glucose in patients with type Dosage/Administration: ORAL: For type 1 or type 2 diabetes, 1 to 6 grams (1 teaspoon = 4.75 grams) of cassia cinnamon daily for up to 4 months have been used. (8, 13, 18, 19). Editor's Comments: There are a lot of different types of cinnamon. Cinnamomum verum (Ceylon Specific References: Cinnamon 1. lectronic Code of Federal Regulations. Title 21. Part 182 - 2. Lee HS, Ahn YJ. Growth-Inhibiting Effects of Cinnamomum cassia Bark- 3. Koh WS, Yoon SY, Kwon BM, et al. Cinnamaldehyde inhibits lymphocyte 4. Kwon BM, Lee SH, Choi SU, et al. Synthesis and in vitro cytotoxicity of 5.Anderson RA, Broadhurst CL, Polansky MM, et al. Isolation and 6. Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from 7. Imparl-Radosevich J, Deas S, Polansky MM, et al. Regulation of PTP-1 and 8. 11347 Khan A, Safdar M, Ali Khan M, et al. Cinnamon improves glucose and 9. De Benito V, Alzaga R. Occupational allergic contact dermatitis from cassia 10.Drake TE, Maibach HI. Allergic contact dermatitis and stomatitis caused by a 11.Onderoglu S, Sozer S, Erbil KM, et al. The evaluation of long-term effcts 12. 3238 13. 14. ress release. Cinnamon capsules to reduce blood sugar are medicinal 15.Miller KG, Poole CF, Pawloski TMP. Classification of the botanical origin 16.He ZD, Qiao CF, Han QB, et al. Authentication and quantitative analysis on 17.Felter SP, Vassallo JD, Carlton BD, Daston GP. A safety assessment of 18.Baker WL, Gutierrez-Williams G, White CM, et al. Effect of cinnamon on 19. Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in 20. Blevins SM, Leyva MJ, Brown J, et al. Effect of cinnamon on glucose and lipid | |
Cleavers
Bedstraw, Catchweed, Cleavers, Gallium, Goose Grass, Gosling Weed, Robin-Run-in-the-Grass, Scratchweed, Stick-a-Back, Sticky Willy. Scientific Name: Galium aparine. Family: Rubiaceae. People Use This For: Clivers is used as a diuretic, a mild astringent, for dysuria, lymphadenitis, psoriasis, and specifically for enlarged lymph nodes. Safety: No concerns regarding safety when used orally and appropriately.13 There is no documented toxicity.14 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available about the effectiveness of clivers. Mechanism of Action: The applicable parts of clivers are the dried or fresh above ground parts. Cleavers contain tannins, which are reported to have astringent properties.14 Adverse Reactions: None reported. Interactions with Herbs & Supplements: None known. Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: None known. Dosage/Administration: Dr Clare’s Blends: 1gm per day. Oral: Typical doses are 2-4 grams dried above ground parts three times daily, or one cup tea (steep 2-4 grams herb in 150 mL boiling water 5-10 minutes, strain) three times daily.14 Liquid extract (1:1 in 25% alcohol) 2-4 mL three times daily.14 Spedific References: CLIVERS 13. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 14. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. | |
CORE REFERENCES
R5. Grases, F., March, J. G., Ramis, M. & Costa-Bauzá, A. (1993) The influence of Zea mays on urinary risk factors for kidney stones in rats.
| |
Cornsilk
Indian Corn, Yu Mi Xiu, Zea. Scientific Name: Zea mays. Family: Poaceae/Gramineae. People Use This For: Orally, corn silk is used for cystitis, urethritis, bedwetting, inflammation of the prostate, acute and chronic inflammation of the urinary system. Safety: No concerns regading safety, available studies validate this statement, when used orally in amounts commonly found in foods. Generally Recognized as Safe (GRAS) status in the US.6 No concerns regarding safety when used orally and appropriately in medicinal amounts.7 Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: There is not enough scientific information available to comment about the effectiveness of corn silk. Mechanism of Action: Corn silk contains tannins, which are astringent, and cryptoxanthin, which has vitamin A activity.8 Diuretic. Demulcent (mucilagenous). Choleretic (promotes bile flow). The diuretic and choleretic action has been demonstrated in animal studies.R5 In China it is used for Hepato-Biliary Disease.R6,R7 Adverse Reactions: None. Interactions with Herbs & Supplements: Herbs and Supplements with Hypotensive Effects: Corn silk is thought to have hypotensive effects, may have an additive effect with blood pressure lowering agents. Interactions with Drugs: Antidiabetes Drugs: Theoretically, because some evidence suggests corn silk can reduce blood glucose levels, excessive amounts might interfere with diabetes therapy.R1 pp.222 Diuretic Drugs: Additive Effect.R4 pp.192,204,215 Lithium: Because of Diuretic effect. Warfarin (Coumadin): Corn silk contains vitamin K. Individuals taking warfarin should consume a consistent daily amount to maintain consistent anticoagulation.9 Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None within normal dose range. Dosage/Administration: Oral: 4-8 grams dried style/stigma three times daily, or one cup tea (steep 0.5 grams dried corn silk in 150 mL boiling water 5-10 minutes, strain) several times daily.8,10 Liquid extract of maize stigmas, 4-8 mL.8 Tincture (1:5 in 25% alcohol), 5-15 mL three times daily. 8 Syrup of maize stigmas, 8-15 mL.8 Dr Clare’s Comments: Corn silk is the silky threads that surround the ear of sweetcorn. It is one on the few herbs that are more effective dried, probably because they have such a high water content it would take a lot of them to make an effective fresh infusion. It would be nice to think that Corn Silk would ‘treat’ high blood pressure/diabetes however it is likely to be helpful in the way an extra portion of fruit or vegetables are helpful. It will not reduce a normal blood sugar or a normal Blood Pressure in my experience. Nor has it been reported, these are theoretical considerations. Specific References: CORN SILK 6. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 7. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 8. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 9. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. 10. Stuttgart: Medpharm GmbH Scientific Publishers, 1994 | |
Couchgrass
Agropyron, Coughgrass, Cutch, Dog Grass. Scientific Name: Agropyron repens. Family: Poaceae/Gramineae. People Use This For: It is used orally to treat cystitis, urethritis, prostatitis, benign prostatic hypertrophy and kidney stones. Safety: No concerns regarding safety, available studies validate this statement, when consumed in amounts commonly found in foods.11 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: Not enough scientific information gather to offer a comment. Mechanism of Action: Diuretic.R8 pp.18,R1 pp.222 Adverse Reactions: None known Interactions with Herbs & Supplements: None known. Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None known. Dosage/Administration: Oral: For treating ulcerative colitis, 100 mL of wheatgrass juice daily for 1 month has been used.12 DrClare’s Blends: Specific References: COUCHGRASS 11. Rauma AL, Nenonen M, Helve T, et al. Effect of a strict vegan diet on energy and nutrient intakes by Finnish rheumatoid patients. Eur J Clin Nutr 1993;47:747-9. 12. Ben-Arye E, Golden E, Wengrower D, et al. Wheat grass juice in the treatment of active distal ulcerative colitis a randomized double-blind placebo-controlled trial. Scand J Gastroenterol 2002;4:444-9. | |
Cramp Bark
Also known as: Common Guelder-Rose, Cranberry Bush. Scientific Name: Viburnum opulus. Botanical Family: Adoxaceae/Viburnaceae (formerly known as Caprifoliaceae). Part used: Bark and root bark.
Traditional Use. Cramp bark has antispasmodic (relieves muscle spasms), anti-inflammatory (relieves inflammation), nervine (calms and soothes the nerves), hypotensive (lowers blood pressure), astringent (causes local contraction), emmenagogic (induces menstruation ), and sedative (reduces activity and excitement) properties.
Safety. There are no reports of safety concerns regarding Cramp Bark.
Pregnancy: Refer to a Medical Herbalist. Breastfeeding: Refer to a Medical Herbalist. Constituents Hydroquinines; arbutin, methylarbutin and traces of free hydroquinone. Sesquiterpene dialdehyde fraction; viopudiol. Coumarins; such as scopoletinans, esculatin. Triterpinoids; including oleanic acid and ursolic acid derivatives. Iridoid glysoside esters.
Scientific evidence. No clinical research has been done. Mechanism of action. At least two active constituents have been identified, scopoletin and viopudial. These constituents appear to have smooth muscle antispasmodic effects in vitro. Viopudial appears to have cholinergic effects by inhibiting acetylcholinesterase. The effect opposes the dominence of the sympathetic nervous system and relaxes the tone in smooth muscles. There is evidence of anti-inflammatory effects.
One study showed that the administration of viopudial, a Viburnum opulus component, produces slowing of the heart rate, lowering of blood pressure, and some decrease in contractility of heart muscle. This action is by balancing the sympathetic and parasympathetic nervous system tone in the body. Experimental evidence indicates that viopudial's mechanism of action is partly due to its effects on cholinesterase. In vitro demonstrations of a competitive inhibitory effect on both acetylcholinesterase and butyrylcholinesterase showed viopudial to be relatively weak when compared to the known potent inhibitor, physostigmine. Additional mechanistic effects, such as a direct musculotrophic action, may also be responsible for the overall activity. 8 One animal study shows that proanthocyanidin constituents of Viburnum opulus exert a potent gastro-duodenoprotective effect by increasing nitrous oxide in the tissues, suppressing lipid peroxidation and mobilizing antioxidant activity and changes in the gastroduodenal mucosa of rat.7 Other studies show that Viburnum opulus exhibits anti-oxidant effects.9
Adverse reactions. None reported.
Possible interactions with herbs and supplements. None known.
Possible interactions with drugs. None known.
Possible interactions with foods. None known.
Interactions with laboratory tests. None known.
Effects on diseases or conditions. None known.
Dosage. Recommended dose: 3-10mls per day 1:5 tincture 30% alcohol. Decoction: range from ½ to 6 tsps. per day. Powder/capsule: range from 1-4gms per day. Raw herb: 2-4gms per day.R8 pp.230 Liquid extract: 2-8ml/day.
The recommended dose of Dr Clare’s Joint Support Blend provides 3mls per day of 1:3 Tincture in 15mls daily, at a dose of 5mls three times a day. This is equivalent to 375-750mgs per day.
References. 1. Exp Biol Med (Maywood) June 1972 vol. 140 no. 2 457-461 Viopudial, a Hypotensive and Smooth Muscle Antispasmodic from Viburnum opulus.John A. Nicholson,Thomas D. Darby, Charles H. Jarboe
2. Charles H. Jarboe , Karimullah A. Zirvi , John A. Nicholson , Charlotte M. Schmidt. Scopoletin, an Antispasmodic Component of Viburnum opulus and prunifolium J. Med. Chem., 1967, 10 (3), pp 488–489
3. Antioxidant properties of Viburnum opulus and Viburnum lantana growing in Turkey. Dr Mehmet Levent Altun, Gülçin Saltan Çitoğlu, Betül, Sever Yilmaz and Tülay Çoban International Journal of Food Sciences and Nutrition 2008, Vol. 59, No. 3 , Pages 175-180
4. Antinociceptive and Anti-inflammatory Activities of Viburnum lantana. Pharmaceutical Biology2007, Vol. 45, No. 3 , Pages 241-245 B. Sever Yilmaz, G Saltan Citoglu, M.L. Altun and H. Ozbek
5. Zeng LJ, Xing JB, Li P. China Journal of Chinese Materia Medica [2000, 25(3):184-188]
6. Ilkay Erdogan-Orhan, Mehmet Levent Altun, Betül Sever-Yilmaz, and Gülçin Saltan. Anti-Acetylcholinesterase and Antioxidant Assets of the Major Components (Salicin, Amentoflavone, and Chlorogenic Acid) and the Extracts of Viburnum opulus and Viburnum lantana and Their Total Phenol and Flavonoid Contents Journal of Medicinal Food. April 2011, 14(4): 434-440.
7. Zayachkivska OS1, Gzhegotsky MR, Terletska OI, Lutsyk DA, Yaschenko AM, Dzhura OR. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J Physiol Pharmacol. 2006 Nov;57 Suppl 5:155-67.
8. Viopudial, a hypotensive and smooth muscle antispasmodic from Viburnum opulus. J A Nicholson, T D Darby, C H Jarboe Proceedings of The Society for Experimental Biology and Medicine 07/1972; 140(2):457-61.
9. Andreeva T.I, Komarova E.N, Yusubov M. S, Korotkova E.I. Antioxidant activity of cranberry tree (Viburnum Opulus L.) bark extract. Pharmaceutical Chemistry Journal 10-2004, Volume 38, Issue 10, pp 548-550
| |
D |
|---|
Dandelion Root
Also known as: Blowball, Common Dandelion, Dent-de-Lion (Lions tooth). Scientific Name: Taraxacum officinale radix. Botanical Family: Asteraceae/Compositae. Part used: Root (dandelion leaves are also used but this profile relates to the root). Traditional use. Dandelion is used for loss of appetite, dyspepsia, flatulence, gallstones, bile stimulation, rheumatism, arthritic joints, muscle aches, eczema, and bruises. Dandelion is also used as an alterative, laxative, diuretic, circulatory tonic, skin toner, blood tonic, and digestive tonic. It is also used to treat infection, especially viral infections. In foods the roasted root is used as a coffee substitute. Nutrients: dandelion is a rich source of vitamins A, B complex, C, and D, as well as minerals such as iron, potassium, and zinc. The root is generally richer in minerals than the leaf which is rich in vitamins. Safety. There are no concerns regarding safety when used orally in amounts commonly found in foods. Dandelion has Generally Recognized As Safe (GRAS) status in the US.(1) There are no concerns regarding safety when used appropriately in medicinal amounts, see dosage information for guidance.(2) Pregnancy: There are no scientific studies available, so avoid using greater than dietary amounts. Breastfeeding: There are no scientific studies available, so avoid using greater than dietary amounts. Constituents Sesquiterpene lactones; tataxoside, taraxinic acid, dihydrotaraxininc acid and others. Polyphenols including caffeic acid Coumarins Triterpenes; tataxol, taraxerol, tataxasterolbeta-amyrin, stigmasterol and beta-sitosterol (a phytosterol) Carbohydrates especially inulin when harvested in Autumn) Vitamins A, B, C, D Minerals especially potassium and calcium Scientific evidence. There is insufficient scientific data to comment on most traditional uses. Urinary tract infections (UTIs). A specific oral combination of dandelion root and uva ursi leaf extracts seems to help reduce the recurrence rate of UTIs in women.(3) In this combination uva ursi is used for its antibacterial properties and dandelion is used to increase urination. Mechanism of action. Dandelion root contains quercetin, luteolin, p-hydroxyphenylacetic acid, germacranolide acids, chlorogenic acid, chicoric acid, and monocaffeyltartaric acid. In addition it has a high potassium content. The roots contain caffeic acid, taraxacoside, taraxasterol, and large amounts of the polysaccharide, inulin.(4) Dandelion root promotes bile production and bile flow and urinary flow. It is anti-inflammatory, anti-oxidative, analgesic, anti-hyperglycemic, anti-coagulatory and has prebiotic effects. Based on a small study using an alcohol extract of dandelion leaf in human volunteers, this herb elicits a significant diuretic effect in humans.(5 Emerging evidence suggests that dandelion and its constituents have antioxidant and anti-inflammatory activities that result in diverse biological effects.(6,7,13) Animal studies show that dandelion has effects on detoxifying enzymes in the liver.(9) Preliminary evidence indicates a beneficial effect on cardiovascular risk factors mediated by oxidative stress, inflammation, and cholesterol profile. (13) Dandelion root contains high concentrations of inulin. Oligofructans, such as inulin, are used as food sources by beneficial intestinal bacteria. Dandelion root enhances the growth of bifidobacteria and may be useful as a "prebiotic".(8) Dandelion extracts were effective for facilitating the gastrointestinal motility in animal studies.(12) Anti-cancer effects on melanoma, breast and prostate cancer cells have been demonstrated. (10,11) Adverse reactions. No reported side effects. Possible interactions with herbs and supplements. None known. Interactions with drugs. Lithium; Seek professional advice when combining any herbs, spices or foods that have a diuretic effect so that accommodation with the monitoring and dosage of Lithium can be adjusted. Interactions with foods. None known. Interactions with laboratory tests. None known. Dosage. Recommended dose: 5-10mls per day 1:5 tincture 30% alcohol. Decoction: range from 3-6 tsps. per day. Tincture; 5-10 mls per day 1:5 25% alcohol Raw herb: 3-5gms per day Juice from fresh root: 3-8mls per day. Liquid extract: The recommended dose of Dr Clare’s Joint Support Tea provides ⅙ of a tsp. three times a day or ½ a tsp. per day. Dr Clare’s Blend: 1gm per day Liquid extract: 2-8 mL / day Tincture: 1:5, 5-10 mL / day Root tincture (1:2) fresh root in 45% alcohol: 30 - 60 drops, 3 times daily. Raw Herb: Usual dose; 2-8gms/day. Dried root decoction: 1/2 - 2 teaspoonfuls, 3 times daily. Place throot into boiling water for 5 - 10 minutes. Strain and drink the ‘tea’. You can add the roots to soups or stews, they are very nourishing. References. 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 2. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 3. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res 1993;53:441-3. 4. Williams CA, Goldstone F, Greenham J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996;42:121-7. 5. Bevin A. Clare, Richard S. Conroy, Kevin Spelman. The Diuretic Effect in Human Subjects of an Extract of Taraxacum officinale Folium over a Single Day. The Journal of Alternative and Complementary Medicine. August 2009, 15(8): 929-934. 6. Gonzalez-Castelon M, Visioli F, Rodriguez-Casado A. Diverse biological activities of dandelion. Nutr Rev. 2012 Sep;70(9):534-47. 7. Mascolo N, Autore G, Capassa G, et al. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res 1987:28-9. 8. Trojanova I, Rada V, Kokoska L, Vlkova E. The bifidogenic effect of Taraxacum officinale root. Fitoterapia 2004;75:760-3. 9. Maliakal PP, Wanwimolruk S. Effect of herbal teas on hepatic drug metabolizing enzymes in rats. J Pharm Pharmacol 2001;53:1323-9. 10. The Efficacy of Dandelion Root Extract in Inducing Apoptosis in Drug-Resistant Human Melanoma Cells 11. Sigstedt SC, Hooten CJ, Callewaert MC, Jenkins AR, et al. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int J Oncol. 2008 May;32(5):1085-90. 12. S. J. Chatterjee, P. Ovadje, M. Mousa, C. Hamm, and S. Pandey. Evidence-Based Complementary and Alternative Medicine. Volume 2011.Research on the gastrointestinal propulsive motility and chemical constituents of Dandelion extraction WU Yan-ling, PIAO Hui-shan. 13. Jinju Kim , Kyunghee Noh , Mikyung Cho , Jihyun Jang and Youngsun Song. Anti-oxidative, anti-inflammatory and anti-atherogenic effects of Dandelion (Taraxacum officinale) extracts in C57BL/6 mice fed atherogenic diet Jinju Kim , Kyunghee Noh , Mikyung Cho , Jihyun Jang and Youngsun Song. Journal of Fed. of American Societies for Experimental Biology 2007;21:862.7
| |
Devil’s Claw
Also known as: Grapple Plant. Scientific name: Harpagophytum procumbens. Botanical Family: Pedaliaceae. Part used: The tubers which are underground stems. Traditional use. Devil's claw is traditionally used for arteriosclerosis, osteoarthritis, rheumatoid arthritis, gout, muscle soreness, fibrositis, lumbago, tendonitis, pleuritic chest pain, gastrointestinal upset or dyspepsia, fever, migraine headache, menstrual problems, allergic reactions, loss of appetite, kidney and bladder disease, and degenerative disorders of the locomotor system. Safety. No concerns regarding safety when used orally and appropriately. Devil's claw seems to be well-tolerated when used daily for up to a year.(1,2,3,4,5) Pregnancy: Consult a medical herbalist. Breastfeeding: Consult a medical herbalist. Constituents Iridoid glycosides principally harpagoside Sugars. Triterpenes. Phytosterols especially beta sitosterol. Aromatic acids. Flavonoids; kaemferol and lutein. Harpagoquinone Scientific evidence. Back pain. Taking devil's claw orally seems to lessen nonspecific low-back pain. Some evidence suggests that an aqueous extract of devil's claw at doses of 50-100mg harpagoside daily has an anti-inflammatory effect equal to 13.5mg rofecoxib (Vioxx).(2,3,5) If the resources put into even the marketing budget of Vioxx was spent investigating this herb the tragedy of the Vioxx deaths and ilness may not have happened. Osteoarthritis. Taking devil's claw orally alone or in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) helps decrease osteoarthritis-related pain.(1,2,3,4,13) Evidence shows that devil's claw is comparable to diacerhein (a slow-acting drug for osteoarthritis; not available in the US) for improving osteoarthritis pain in the hip and knee after 16 weeks of treatment. Patients taking devil's claw have been able able to decrease the use of NSAIDs for pain relief.(1) This study used a specific powdered devil's claw root product (Harpadol, Arkopharma) containing 2% of the constituent harpagoside (9.5mg/capsule) and 3% total iridoid glycosides (14.5mg per capsule).(1) Another specific devil's claw extract (Doloteffin, Ardeypharm) 2400mg/day providing 60mg/day of the harpagoside constituent has also been researched.(2,4) Rheumatoid arthritis (RA). Preliminary evidence suggests that taking devil's claw extract orally might not be helpful for RA.(6) More evidence is needed to rate devil's claw for this use. Mechanism of action. Devil's claw contains iridoid glycoside constituents primarily harpagoside but it appears that other compounds besides harpagoside contribute to its effect.(7,8) It also contains the phenylethanol derivatives and an oligosaccharide.(8) People use devil's claw for osteoarthritis and other inflammatory conditions because the iridoid glycoside constituents seem to have an anti-inflammatory effect.(1) Some preliminary research suggests that harpagoside inhibits both the cyclooxygenase (COX) and lipoxygenase inflammatory pathways.(9) Devil's claw seems to inhibit COX-2 (but not COX-1) and nitric oxide synthetase, a modulator of inflammation.(10) Some evidence suggests that the anti-inflammatory effect is due to increased synthesis and release of tumor necrosis factor by compounds other than harpagoside.(8) However, research in humans shows no effect of devil's claw on the arachidonic acid pathway.(11) Adverse reactions. Devil's claw is generally well tolerated. A small percentage of patients complain of gastrointestinal upset. This can only be determined by trying the herb. It is more likely in patients wih peptic ulceration. In general if you have digestive problems start with a lower than usual dose and increase the dose slowly to test your tolerance level. Devil's claw can cause allergic skin reactions following oral treatment. Although references cite that devil’s claw may lower blood sugar levels, upset gallstones and may affect blood pressure, these are extrapolations from physiological effects or animal studies and I can find no clinical studies or even case reports of problems. Be aware that herbs alter physiology and monitor any chronic condition for change when you use herbs. Interactions with herbs and supplements. None known. Interactions with drugs. Warfarin (Coumadin) an anti blood clotting agent. Interactions with foods. None known. Interactions with laboratory tests. None known. Interactions with diseases or conditions. Peptic Ulcer Disease: May cause gastrointestinal upset.12 However lower doses may be clinically indicated and well tolerated for digestive discomfort Dosage. Recommended dose: 6-12mls per day 1:5 tincture 25% alcohol. Liquid extract: 1-2mls 1:1 25% alchohol. Decoction: range from 2-4 tsps. per day. Powder/capsule: 1,500mgs per day, The recommended dose of Dr Clare’s Joint Support Blend provides 3mls per day of 1:3 Tincture. This is when taken at a dose of 5mls three times a day. This is equivalent 750mgs per day. References. 1. Chantre P, Cappelaere A, Leblan D, et al. Efficacy and tolerance or Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000;7:177-84. 2. Chrubasik S, Thanner J, Kunzel O, et al. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract doloteffin in patients with pain in the lower back, knee or hip. Phytomedicine 2002;9:181-94. 3. Gagnier JJ, Chrubasik S, Manheimer E. Harpgophytum procumbens for osteoarthritis and low back pain: a systematic review. BMC Complement Altern Med 2004;14:13. 4. Wegener T, Lupke NP. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil's claw (Harpagophytum procumbens DC). Phytother Res 2003;17:1165-72. 5. Chrubasik S, Kunzel O, Thanner J, et al. A 1-year follow-up after a pilot study with Doloteffin for low back pain. Phytomedicine 2005;13:1-9. 6. Grahame R, Robinson BV. Devils's claw (Harpagophytum procumbens): pharmacological and clinical studies. Ann Rheum Dis 1981;40:632. 7. Lanhers MC, Fleurentin J, Mortier F, et al. Anti-inflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med 1992;58:117-23 . 8. Fiebich BL, Heinrich M, Hiller KO, Kammerer N. Inhibition of TNF-alpha synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine 2001;8:28-30.. 9. Chrubasik S, Sporer F, Dillmann-Marschner R, et al. Physicochemical properties of harpagoside and its in vitro release from Harpagophytum procumbens extract tablets. Phytomedicine 2000;6:469-73. 10. Jang MH, Lim S, Han SM, et al. Harpagophytum procumbens suppresses lipopolysaccharide-stimulated expressions of cyclooxygenase-2 and inducible nitric oxide synthase in fibroblast cell line L929. J Pharmacol Sci 2003;93:367-71. 11. Moussard C, Alber D, Toubin MM, et al. A drug used in traditional medicine, harpagophytum procumbens: no evidence for NSAID-like effect on whole blood eicosanoid production in human. Prostaglandins Leukot Essent Fatty Acids. 1992;46:283-6. 12. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. 13. Effectiveness and safety of Devil's Claw tablets in patients with general rheumatic disorders. Phytotherapy Research Volume 21, Issue 12, pages 1228–1233, December 2007 | |
Dong Quai
Angelica sinensis, Chinese Angelica, Dang Gui. Scientific Name: Angelica sinensis. Family: Apiaceae/Umbelliferae. People Use This For: Orally, dong quai is used for painful periods, premenstrual syndrome (PMS), and menopausal symptoms. It is also used orally as a "blood purifier"; to manage high blood pressure, infertility, rheumatism, ulcers, anemia, and constipation; and in the prevention and treatment of allergic attacks. Dong quai is also used orally is for the treatment of skin depigmentation and psoriasis. Safety: No converns regarding safety when used orally and appropriately. Dong quai has been safely used in a clinical trial lasting up to 24 weeks.34 More scientific evidence is needed to determine its safety after prolonged, repetitive use. Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: POSSIBLY EFFECTIVE Men suffering from premature ejaculation who were treated with the cream had significantly improved ejaculatory latency compared to placebo.35 POSSIBLY INEFFECTIVE Menopausal symptoms. Dong quai does not seem to have any effect on endometrial wall thickness or menopausal symptoms.34 There is insufficient reliable information available about the effectiveness of dong quai for its other uses. Mechanism of Action: The applicable part of dong quai is the root. Dong quai root has several active constituents. Some of these include ferulic acid, ligusticide, angelicide, brefeldin A, butylphthalide, nicotinic acid, and succinic acid.36,37 Dong quai root contains a variety of other constituents. The dong quai root also contains several vitamins and minerals including vitamin A, carotenoids, vitamin E, vitamin C, vitamin B12, biotin, folic acid, calcium, magnesium, and phytosterols such as beta- sitosterol.37 It also contains 0.4% to 0.7% volatile oil.38,37 Dong quai also contains several coumarin constituents.38,37 Some coumarins can act as vasodilators and antispasmodics.
Adverse Reactions: Dong quai is well-tolerated.34
Interactions with Drugs: Warfarin (Coumadin): Refer to a Medical Herbalist.
Interactions with Foods: None known.
Interactions with Diseases or Conditions: Breast Cancer: Refer to a Medical Herbalist. Hormone Sensitive Cancers/Conditions: Refer to a Medical Herbalist. Surgery: Tell patients to discontinue dong quai at least 2 weeks before elective surgical procedures. Oral: For menopausal symptoms, 4.5 g of powdered Dong Quai root has been used daily.34
Specific References: DONG QUAI 34. Hirata JD, Swiersz LM, Zell B, et al. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6. 35. Choi HK, Jung GW, Moon KH, et al. Clinical study of SS-Cream in patients with lifelong premature ejaculation. Urology 2000;55:257-61. 36. Zhao KJ, Dong TT, Tu PF, et al. Molecular genetic and chemical assessment of radix Angelica (Danggui) in China. J Agric Food Chem 2003;51:2576-83. 37. Monograph. Angelica sinensis (Dong quai). Altern Med Rev 2004;9:429-33. 38. Zhu DP. Dong Quai. Am J Chin | |
E |
|---|
Echinacea
American Cone Flower, Échinacée Angustifolia, Échinacée Pallida, Échinacée Purpurea, Purple Cone Flower. Scientific Name: Echinacea angustifolia; Echinacea pallida; Echinacea purpurea. Family: Asteraceae/Compositae. People Use This For: Orally, echinacea is used for treating and preventing the common cold and other upper respiratory infections. Echinacea is also used orally as an immunostimulant for fighting a variety of other infections, including urinary tract infections (UTIs), vaginal candidiasis (yeast infections), and genital herpes (HSV Type 1 and 2). Echinacea is also used orally for nasal cattarh, allergic rhinitis, gum disease and tonsillitis. Other uses include chronic fatigue syndrome (CFS), rheumatism, migraines, dyspepsia, pain, dizziness, rattlesnake bites and swine flu. Topically, echinacea is used for boils, abscesses, skin wounds and ulcers, burns, eczema, psoriasis, UV radiation skin damage, herpes simplex, bee stings, and hemorrhoids. Safety: No concerns regarding safety when used orally and appropriately, short-term. Available studies validate this statement. Several formulations of echinacea have been used safely in trials lasting up to 12 weeks.17,18,19,20,21,22,23,24,25,26,27,28 There is not enough scientific evidence on the safety of long term use of echinacea to comment. There is no evidence of harm. Children: Possibly safe. There is evidence from research that an Echinacea purpurea juice extract is safe in children aged 2-11 years when used for up to 10 days. However, echinacea might increase the risk of rash in some children.29,26 Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: POSSIBLY EFFECTIVE Common cold. Taking some echinacea preparations seems to modestly reduce symptom severity and duration, possibly by about 10% to 30%.17,19,32,33,37,21,22,24,25,27,28 Echinacea seems to be most effective if started when symptoms are first noticed and continued for 7-10 days. Not all research is positive. Some studies show no benefit for treating the common cold in adults.20,23,47,55,28 A study in children aged 2-11 years also suggests that taking an Echinacea purpurea juice extract 7.5-10 mL/day (Madaus AG, Germany) for up to 10 days also does not significantly decrease cold symptoms.29 Taking echinacea prophylactically to prevent the development of a cold does not seem to be effective.19,20,34,21,56,57,50,55,28 Echinacea studies have used different echinacea species and a wide variety of preparation methods. Studies have also used different patient populations and study designs. Due to these discrepancies among studies, it's not surprising that different studies have different results.23,31,58,30,27 The best evidence (and the most research) appears to be for preparations of the Echinacea purpurea species.28 Other preparations that have been used include a variety of extracts of the herbs and root parts of Echinacea pallida and Echinacea angustifolia species.17,18,19,21,53 Echinacea teas and fixed combination herbal preparations containing echinacea have also been used.19,32,37 Vaginal candidiasis. Taking echinacea orally in combination with a topical antifungal cream seems to be effective for preventing recurrent vaginal yeast infection. Herb juice of Echinacea purpurea in combination with topical econazole (Spectazole) lowers recurrence rate to 16.7% compared to 54.5% with econazole alone.54 POSSIBLY INEFFECTIVE Herpes simplex virus (HSV). Taking echinacea orally doesn't seem to prevent or treat recurrent genital herpes infection. A specific Echinacea purpurea extract (Echinaforce by Bioforce AG) 800 mg twice daily for six months does not seem to prevent or reduce frequency or duration of recurrent genital herpes in patients with herpes simplex virus (HSV) type 1 or 2.41 INSUFFICIENT RELIABLE EVIDENCE to COMMENT Influenza. Taking echinacea orally might modestly reduce some influenza symptoms. However, there is not enough specific evidence to know if echinacea is effective for influenza.19,32,33,34 Mechanism of Action: The parts of echinacea are the roots and the above ground parts. The composition of each of the three commonly used echinacea species is similar, with some variation in the amounts of active constituents. Although these species are often used interchangeably, there is very little research comparing them.38 Echinacea increases the assimilation of detritus into mopping up cells (phagocytosis) and increases lymphocyte (white blood cell) activity, possibly by promoting the release of tumor necrosis factor, interleukin, and interferon.35,36,39 Several constituents of echinacea seem to be involved in stimulating this non-specific immune response. However, echinacea doesn't seem to have any effect on the immune system of healthy volunteers.40 Echinacea's effect on cold symptoms might result from anti-inflammatory activity. Clinical research also suggests an anti-inflammatory effect. Echinacea is also reported to have antifungal properties, so people use it for yeast infections (vaginal candidiasis). Compounds in echinacea seem to have antifungal activity, including activity against Candida yeast.42 For wound healing echinacea seems to protect collagen from free radical damage. It also may have activity against bacterial hyaluronidase. Animal research suggests that echinacea extracts can speed would healing, enhance formation of new skin, and reduce inflammation.38 Preliminary information suggests that echinacea might help treat or prevent UV radiation skin damage by protecting collagen from free radical damage.43 Preliminary research also suggests that high concentrations of Echinacea purpurea might reduce sperm and ova fertility.44,45 Adverse Reactions: Orally, echinacea is usually well-tolerated by most people.17,18,20,46,47,26 Gastrointestinal adverse effects, allergic reactions, fever, heartburn, constipation, unpleasant taste, dry mouth, sore throat, tingling sensation and numbness of the tongue, mouth ulcers, headache, dizziness, insomnia, and disorientation.48,47,50,25,26 Arthralgia and myalgia have also been associated with Echinacea.26 Allergic reactions can include urticaria; erythema nodosum;51 itchy, watery eyes; runny nose;48 chest tightness; dyspnea; bronchospasm; acute asthma; facial and upper airway angioedema; and anaphylaxis.49,52,48 In a study of children aged 2-11 years, about 7% of children experienced a rash after taking Echinacea compared with 2.9% of those taking placebo. This has not been borne out by other studies. This may have been caused by an allergic reaction.29 or it may have been the rash was part of the illness for which the children were taking Echinacea. Allergic reactions seem to be uncommon.52,48 Interactions with Herbs & Supplements: None known. Interactions with Drugs: Immunosuppressants: Refer to a Medical Herbalist. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: Atopy: Individuals a genetic tendency toward allergic conditions may be more likely to experience an allergic reaction when taking echinacea. It is not a reason not to use Echinacea unless a problem has been noted. Autoimmune Diseases: Refer to a Medical Herbalist. Dosage/Administration: Dr Clare’s Blends: 1 gm per day Echinacea purpurea Oral: A wide variety of dosages and forms have been used. This is one reason why it is so difficult to interpret research data. Topical: No typical dosage. Dr Clare’s Comment. In ten years of regular prescribing of Echinacea I have not seen an allergic reaction. It is very well tolerated in traditional doses; I have never had to stop Echinacea because of side effects. Specific References: ECHINACEA 17. Brinkeborn RM, Shah DV, Degenring FH. Echinaforce and other Echinacea fresh plant preparations in the treatment of the common cold. A randomized, placebo controlled, double-blind clinical trial. Phytomedicine 1999;6:1-6. 18. Gunning K. Echinacea in the treatment and prevention of upper respiratory tract infections. West J Med 1999;171:198-200. 19. Barrett B, Vohmann M, Calabrese C. Echinacea for upper respiratory infection. J Fam Pract 1999;42:628-29. 20. Grimm W, Muller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med 1999;106:138-37. 21. Giles JT, Palat CT III, Chien SH, et al. Evaluation of Echinacea for treatment of the common cold. Pharmacother 2000;20:690-7. 22. Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2:CD000530. 23. Barrett BP, Brown RL, Locken K, et al. Treatment of the common cold with unrefined echinacea. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2002;137:939-40. 24. Schulten B, Bulitta M, Ballering-Bruhl B, et al. Efficacy of Echinacea purpurea in patients with a common cold. A placebo-controlled, randomised, double-blind clinical trial. Arzneimittelforschung 2001;45:563-8. 25. Goel V, Lovlin R, Barton R, et al. Efficacy of a standardized echinacea preparation (Echinilin) for the treatment of the common cold: a randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther 2004;29:75-83. 26. Huntley AL, Thompson Coon J, Ernst E. The safety of herbal medicinal products derived from Echinacea species: a systematic review. Drug Saf 2005;28:387-400. 27. Caruso TJ, Gwaltney JM Jr. Treatment of the common cold with echinacea: a structured review. Clin Infect Dis 2005;34:807-10. 28. Linde K, Barrett B, Wolkart K, et al. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2006;(1):CD000530. 29. Taylor JA, Weber W, Standish L, et al. Efficacy and safety of echinacea in treating upper respiratory tract infections in children: a randomized controlled trial. JAMA 2003;290:2824-30. 30. Krochmal R, Hardy M, Bowerman S, et al. Phytochemical assays of commercial botanical dietary supplements. Evid Based Complement Alternat Med 2004;1:305-3. 31. Turner RB. Echinacea for the common cold: can alternative medicine be evidence-based medicine? Ann Intern Med 2002;137:1001-2. 32. Lindenmuth GF, Lindenmuth EB. The efficacy of echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double-blind, placebo-controlled study. J Altern Complement Med 2000;6:327-28. 33. Dorn M, Knick E, Lewith G. Placebo-controlled, double-blind study of Echinaceae pallidae radix in upper respiratory tract infections. Complement Ther Med 1997;5:34-2. 34. Melchart D, Walther E, Linde K, et al. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med 1998;7:541-5. 35. Luettig B, Steinmuller C, Gifford GE, et al. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst 1989;81:669-75. 36. Stimpel M, Proksch A, Wagner H, et al. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun 1984;40:845-9. 37. Henneicke-von Zepelin H, Hentschel C, Schnitker J, et al. Efficacy and safety of a fixed combination phytomedicine in the treatment of the common cold (acute viral respiratory tract infection): results of a randomised, double blind, placebo-controlled, multicentre study. Curr Med Res Opin 1999;15:214-21. 38. Speroni E, Govoni P, Guizzardi S, et al. Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J Ethnopharmacol 2002;79:265-72. 39. Barrett B. Medicinal properties of Echinacea: a critical review. Phytomedicine 2003;10:60-86. 40. Schwarz E, Metzler J, Diedrich JP, et al. Oral administration of freshly expressed juice of Echinacea purpurea herbs fail to stimulate the nonspecific immune response in healthy young men: results of a double-blind, placebo-controlled crossover study. J Immunother 2002;19:413-20. 41. Vonau B, Chard S, Mandalia S, et al. Does the extract of the plant Echinacea purpurea influence the clinical course of recurrent genital herpes? Int J STD AIDS 2001;12:154-8. 42. Binns SE, Purgina B, Bergeron C. Light-mediated antifungal activity of Echinacea extracts. Plant Med 2000;60:241-4. 43. Facino RM, Carini M, Aldini G, et al. Echinacoside and caffeoyl conjugates protect collagen from free radical-induced degradation: a potential use of echinacea extracts in the prevention of skin photodamage. Planta Med 1995;55:510-4. 44. Ondrizek RR, Chan PJ, Patton WC, King A. Inhibition of human sperm motility by specific herbs used in alternative medicine. J Assist Reprod Genet 1999;16:87-91. 45. Ondrizek RR, Chan PJ, Patton WC, King A. An alternative medicine study of herbal effects on the penetration of zona-free hamster oocytes and the integrity of sperm deoxyribonucleic acid. Fertil Steril 1999;71:517-22. 46. Parnham MJ. Benefit-risk assessment of the squeezed sap of the purple coneflower (Echinacea purpurea) for long-term oral immunostimulation. Phytomedicine 1996;3:95-102. 47. Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med 2004;164:1237-35. 48. Mullins RJ, Heddle R. Adverse reactions associated with echinacea: the Australian experience. Ann Allergy Asthma Immunol 2002;88:36-45. 49. Mullins RJ. Echinacea-associated anaphylaxis. Med J Aust 1998;168:170-1. 50. Sperber SJ, Shah LP, Gilbert RD, et al. Echinacea purpurea for prevention of experimental rhinovirus colds. Clin Infect Dis 2004;32:1367-71. 51. Soon SL, Crawford RI. Recurrent erythema nodosum associated with echinacea herbal therapy. J Am Acad Dermatol 2001;38:298-9. 52. Mullins RJ. Allergic reactions to Echinacea. J Allergy Clin Immunol 2000;104:S340-341 (Abstract 1003). 53. Percival SS. Use of echinacea in medicine. Biochem Pharmacol 2000;54:155-8. 54. Coeugniet EG, Kuhnast R. Recurrent candidiasis: Adjuvant immunotherapy with different formulations of Echinacin. Therapiewoche 1986;30:3352-8. 55. Turner RB, Bauer R, Woelkart K, et al. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med 2005;353:341-8. 56. Turner RB, Riker DK, Gangemi JD. Ineffectiveness of echinacea for prevention of experimental rhinovirus colds. Antimicrob Agents Chemother 2000;38:1708-9. 57. Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2:CD000530. 58. Anon. Echinacea: cold comfort. Consum Rep 2004;69:30-2. | |
Elderflower
Common Elder, Sambucus. Scientific Name: Sambucus nigra. Family: Adoxaceae/Sambucaceae or Caprifoliaceae. People Use This For: Orally, elderflower is used for sinusitis, cold, influenza (flu), swine flu, bronchitis, and diabetes. It is also used as a laxative for constipation, as a diuretic, to promote sweating, and to stop bleeding. Elderflower preparations are used as a gargle and mouthwash for coughs, colds, laryngitis and flu. It is used on the skin as an astringent. Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Elderflower has Generally Recognized as Safe (GRAS) status in the US.59
No concerns regarding safety when used orally in a specific combination product containing elderflower, gentian root, sorrel verbena, and cowslip flower (SinuComp, Sinupret).60,61 Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: POSSIBLY EFFECTIVE Sinusitis. Taking a specific combination product containing elderflower, gentian root, verbena, cowslip flower, and sorrel (SinuComp Sinupret) seems to help treat acute or chronic sinusitis. Clinical trials have used the brand name product Sinupret.60,61
There is insufficient reliable information available to comment on the effectiveness of elderflower for its other uses.
Mechanism of Action: Elderflower contains lectins, rutin, choline tannin, and lipophilic triterpenoid and sterol compounds such as lupeol and beta-sitosterol.62
Adverse Reactions: Generally well tolerated
Interactions with Herbs & Supplements: None clinically demonstrated.
Interactions with Drugs: None clinically demonstrated.
Interactions with Foods: None known.
Interactions with Lab Tests: None demonstrated
Interactions with Diseases or Conditions: None noted. Dosage/Administration: Dr Clare’s Blends: 1 gm per day
Oral: For acute or chronic sinusitis, a specific combination product (SinuComp Sinupret) containing elderflower 30 mg, plus gentian root 12 mg, and 30 mg each of sorrel, verbena, and cowslip flower has been used three times daily.60,61
Dr Clare’s comment. Very well tolerated, no patient had to stop treatment in 10 years. A very gentle herb suitable for all ages.
Specific References: ELDERFLOWER 59. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 60. Neubauer N, Marz RW. Placebo-controlled, randomized, double-blind, clincal trial with Sinupret sugar coated tablets on the basis of a therapy with antibiotics and decongestant nasal drops in acute sinusitis. Phytomedicine 1994;1:177-81. 61. Marz RW, Ismail C, Popp MA. Action profile and efficacy of a herbal combination preparation for the treatment of sinusitis. Wien Med Wochenschr 1999;149:202-8. 62. Gray AM, Abdel-Wahab YH, Flatt PR. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J Nutr 2000;130:15-20. | |
Eyebright
Augentrostkraut, Eufrasia, Euphrasia, Euphraisia Eye Bright, Euphrasiae herba, Eye Bright, Herbe d'Euphraise. Scientific Name: Euphrasia rostkoviana; Euphrasia officinalis; Euphrasia stricta. People Use This For: Orally, eyebright is used to treat nasal mucous membrane inflammation, allergies, allergic rhinitis, common cold, bronchial conditions, and sinusitis. It is also used orally for cancers, coughs, conjunctivitis, earaches, epilepsy, headaches, hoarseness, inflammation, jaundice, ophthalmia, rhinitis, skin ailments, and sore throat. Safety: POSSIBLY SAFE...when used orally and appropriately (5). ...when used orally in amounts commonly found in foods. Eyebright is listed by the Council of Europe as a natural source of food flavoring (1). Effectiveness: There is insufficient reliable information available about the effectiveness of eyebright. Mechanism of Action: Tannin constituents may be responsible for astringent properties (1). The constituent caffeic acid has bacteriostatic activity (1). Constituents, aucubin and iridoid glycosides, have purgative activity (1). Adverse Reactions: Orally or topically, 10-60 drops eyebright tincture may induce mental confusion, headache, increased eye pressure with lacrimation, itching, redness, swelling of eyelid margins, dim vision, photophobia, weakness, sneezing, nausea, toothache, constipation, cough, dyspnea, insomnia, polyuria, and sweating (1). Interactions with Herbs & Supplements: None known. Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None known. Dosage/Administration: ORAL: 2-4 grams dried above ground parts three times daily (1), or one cup tea (steep 2-4 grams dried above ground parts in 150 mL boiling water 5-10 minutes, strain) three times daily (1). Liquid extract (1:1 in 25% alcohol), 2-4 mL three times daily (1). Tincture (1:5 in 45% alcohol), 2-6 mL three times daily (1). Editor's Comments: Avoid use of nonsterile solutions (including homemade products) in the eye(s), due to high risk of infection. Ophthalmic application of eyebright is not recommended. Historically, eyebright has been used in British Herbal Tobacco, which was smoked for chronic bronchial conditions and colds (2). Specific References: Eyebright
| |
F |
|---|
Fennel
Common Fennel, Garden Fennel, Scientific Name: Foeniculum vulgare. Family: Apiaceae/Umbelliferae. People Use This For: Dyspepsia, flatulence, bloating, loss of appetite, and for colic in infants. It is used for increasing lactation, promoting menstruation, facilitating birth, and increasing libido. Safety: No concerns regarding safety, available studies validate this statement, when used orally in amounts commonly found in foods. Fennel has Generally Recognized as Safe (GRAS) status in the US.17 There is insufficient scientific information available about the safety of fennel when used in medicinal amounts. Children: Insufficient reliable information available. Preliminary clinical research suggests that a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (Colimil) is safe in infants when used for up to a week.18 However, maternal consumption of an herbal tea containing fennel has been linked to neurotoxicity in breast-feeding infants.19 Lactation: Possibly Unsafe when used orally by breast-feeding mothers. Case reports have linked consumption of an herbal tea containing fennel to neurotoxicity in two breast-feeding infants.19 Effectiveness: POSSIBLY EFFECTIVE Colic. A clinical trial shows that breast-fed infants with colic, who are given a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (Colimil) twice daily for a week, have reduced crying times compared to placebo.18
There is insufficient scientific information available about the effectiveness of fennel for other uses. Mechanism of Action: Fennel seed is a rich source of beta-carotene and vitamin C.20 It also contains significant amounts of calcium, magnesium, and iron, and lesser amounts of other metal ions. The seed contains a volatile oil.21 The anethole constituent gives fennel its anise-type aroma and flavor.
Adverse Reactions: Fennel side effects have not been systematically evaluated in clinical research. One clinical trial in infants did not find a significant difference in adverse events compared to placebo.18
Overall low allergic potential except for a small subgroup that cannot tolerate celery, fennel, carrot or mugwort. These are all from the same plant family.R1 pp.383
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: Ciproflozacin (Cipro) Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = D Concomitant use of fennel and ciprofloxacin might reduce the effectiveness of ciprofloxacin. Preliminary evidence suggests that fennel reduces ciprofloxacin bioavailability by nearly 50%, possibly due to the metal cations such as calcium, iron, and magnesium contained in fennel. Evidence also suggests that fennel increases tissue distribution and slows elimination of ciprofloxacin.21
Interactions with Foods: As above
Interactions with Lab Tests: None known.
Dosage/Administration: Oral: No typical dosage. However, traditionally a tea prepared from 1-2 grams of the crushed or ground fruit or seed in 150 mL boiling water has been used. The common dose of the tincture compound is 5-7.5 grams per day. Fennel should be used on a short-term basis. For colic in infants, a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (Colimil) twice daily for a week has been used.18
Dr Clare’s Comments: A very well tolerated herb. Particularly good for wind and bloating. I have not had a patient who had to give up fennel because of side effects. Many people like the taste as it is somewhat sweet. Fennel is native to the Mediterranean, but is now found throughout the world.
Specific References: FENNEL 17. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 18. Savino F, Cresi F, Castagno E, et al. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytother Res 2005;19:335-40. 19. Rosti L, Nardini A, Bettinelli ME, Rosti D. Toxic effects of a herbal tea mixture in two newborns. Acta Paediatrica 1994;83:683. 20. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. 21. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol 1999;51:1391-6. | |
Fenugreek
Also known as: Alholva, Bird's Foot,
Bockshornklee,
Bockshornsame, Chandrika, Egypt Fenugreek, Fenogreco,
Fenugrec, Foenugraeci Semen,
Foenugreek, Greek Clover, Greek
Hay, Greek Hay
Seed, Hu Lu
Ba, Methi, Methika,
Medhika, Sénégrain, Sénégré,
Trigonella, Trigonella Foenum, Trigonelle, Woo Lu Bar. Scientific Name: Trigonella foenum-graecum; Trigonella foenugraecum. Family: Fabaceae/Leguminosae.
People Use This For: Orally, fenugreek is used for diabetes, loss of appetite, dyspepsia, gastroesophageal reflux disease (GERD), gastritis, constipation, atherosclerosis, hyperlipidemia, and for stimulating lactation. Fenugreek is used orally for kidney diseases, beriberi, hernia, and impotence and other male problems. Fenugreek is also used orally for fever, mouth ulcers, boils, bronchitis, cellulitis, tuberculosis, chronic coughs, chapped lips, baldness, and cancer. Topically, fenugreek is used as a poultice for local inflammation, myalgia, lymphadenitis, gout, wounds, leg ulcers, and eczema. In foods, fenugreek is included as an ingredient in spice blends. It is also used as a flavouring agent in imitation maple syrup, foods, beverages, and tobacco. In manufacturing, fenugreek extracts are used in soaps and cosmetics.
Safety: LIKELY SAFE ...when used orally in amounts commonly found in foods. Fenugreek has Generally Recognized as Safe (GRAS) status in the US (5). POSSIBLY SAFE ...when used orally in medicinal amounts ( 8, 13, 16, 17, 12). CHILDREN: POSSIBLY UNSAFE ...when used orally; avoid using. Fenugreek tea has caused loss of consciousness and unusual body odour in children. The body odour may be confused with maple syrup disease (12). PREGNANCY: LIKELY UNSAFE ...when used orally in amounts greater than those found in foods because of its potential oxytocic and uterine stimulant activity (18). Consumption of fenugreek just before delivery may cause the neonate to have an unusual body odour, which could be confused with maple syrup disease. It does not appear to cause long-term sequelae (11). LACTATION: Insufficient reliable information available; avoid using. Although fenugreek is used to promote lactation, there are no clinical studies testing its safety in mother or infant (22).
Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Diabetes. Consuming fenugreek, mixed with food during a meal, seems to reduce postprandial blood glucose levels in patients with type 1 or type 2 diabetes. It may be given in combination with guar gum or by itself (15, 16, 21). Muffins made from a batter consisting of foxtail and barnyard millet, in combination with legumes and fenugreek, do not produce a substantial increase in postprandial blood glucose in diabetic patients (14). Gastroesophageal reflux disease (GERD). Clinical research shows that taking a specific fenugreek product (FenuLife, Frutarom Belgium), 2 grams twice daily 30 minutes before the two biggest meals of the day, significantly improves symptoms of heartburn after one week of treatment and continuing through the 2-week study period. Fenugreek was as effective as taking ranitidine 75 mg twice daily (23). Hypercholesterolemia. There is conflicting evidence about the use of fenugreek for lowering serum cholesterol (8, 13, 16). Hypertriglyceridemia. Preliminary clinical research suggests fenugreek might lower triglycerides in people with type 2 diabetes (21). More evidence is needed to rate fenugreek for these uses.
Mechanism of Action: The applicable part of fenugreek is the seed. The active constituents include trigonelline, 4-hydroxyisoleucine, and sotolon (7, 20).Fenugreek seeds have a distinctive bitter taste and odour. Sotolon is frequently used as a flavoring for artificial maple syrup (20). Soaking fenugreek seeds overnight and washing the seeds in water can decrease some of the taste and odor (13). Fenugreek seeds contain about 50% dietary fiber and pectin and may affect gastrointestinal transit, slowing glucose absorption. About 80% of the total content of free amino acids in the seeds is present as 4-hydroxyisoleucine, which appears to directly stimulate insulin (10, 19, 20, 21). This effect is glucose dependent and only occurs in the presence of moderate to high glucose concentrations. Some evidence suggests the seed consumption might decrease calcium oxalate deposition in the kidneys (4). Fenugreek contains coumarins and other constituents that might affect platelet aggregation, but this might not be significant clinically (7). Preliminary research suggests fenugreek has stimulating effects on the uterus, intestine, and heart (18).
Adverse Reactions: Orally, fenugreek can cause diarrhoea, dyspepsia, abdominal distention, and flatulence (2, 21). With large doses, hypoglycemia is possible (1). Fenugreek can cause allergic reactions including nasal congestion, hoarseness, persistent coughing, wheezing, facial angioedema, and shock (3). The paste of fenugreek applied to the scalp can cause allergic symptoms, including head numbness, facial swelling, and wheezing (3). Consumption of fenugreek by pregnant women just before delivery may cause the neonate to have an unusual body odor, which may be confused with maple syrup disease. It does not appear to cause long-term sequelae (11). This unusual body odor may occur in children drinking fenugreek tea. Loss of consciousness may also occur in children drinking tea made from fenugreek (12).
Interactions with Herbs & Supplements: ANTICOAGULANT/ANTIPLATELET HERBS AND SUPPLEMENTS: Concomitant use of herbs that have constituents that might affect platelet aggregation could theoretically increase the risk of bleeding in some people (6, 7, 8). These herbs include angelica, clove, danshen, garlic, ginger, ginkgo, red clover, turmeric, and others. HERBS AND SUPPLEMENTS WITH HYPOGLYCEMIC POTENTIAL: Theoretically, fenugreek might have additive effects with herbs that decrease blood glucose levels (15, 16). Herbs with hypoglycemic potential include devil's claw, fenugreek, guar gum, Panax ginseng, and Siberian ginseng.
Interactions with Drugs: ANTICOAGULANT/ANTIPLATELET DRUGS <<interacts with>> FENUGREEK Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = B There is some concern that fenugreek might have additive effects when used with anticoagulant or antiplatelet drugs, resulting in increased risk of bruising and bleeding. Some of the constituents in fenugreek have antiplatelet effects, although these might not be present in concentrations that are clinically significant (6, 7, 8). Some drugs with anticoagulant or antiplatelet effects include aspirin, clopidogrel (Plavix), nonsteroidal anti-infl ammatory drugs (NSAIDs) such as diclofenac (Voltaren, Cataflam, others), ibuprofen (Advil, Motrin, others), naproxen (Anaprox, Naprosyn, others), dalteparin (Fragmin), enoxaparin (Lovenox), heparin, and others.
ANTIDIABETES DRUGS <<interacts with>> FENUGREEK Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = B Fenugreek may reduce blood glucose levels (15, 16) and might have additive effects on glucose levels when used with antidiabetes drugs. Monitor blood glucose levels closely. Medication dose adjustments may be necessary. Some antidiabetes drugs include glimepiride (Amaryl), glyburide (DiaBeta, Glynase PresTab, Micronase), insulin, pioglitazone (Actos), rosiglitazone (Avandia), and others.
WARFARIN (Coumadin) <<interacts with>> FENUGREEK Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = D Fenugreek might have additive effects with warfarin and increase the international normalized ratio (INR). Some fenugreek constituents have antiplatelet effects, although these might not be present in concentrations that are clinically significant (7, 8). Fenugreek in combination with boldo has been associated with increased INR in a patient taking warfarin (6).
Interactions with Foods: ALLERGY TO FABACEAE: Chickpea, also a member of the Fabaceae family, has shown cross-reactivity in patients allergic to fenugreek. Theoretically, patients who are allergic to other Fabaceae plants including soybeans, peanuts, and green peas might also be allergic to fenugreek (3). Interactions with Lab Tests: BLOOD GLUCOSE: Fenugreek can lower blood glucose and test results (15, 16). URINE ODOUR: Fenugreek can cause a maple syrup odour in urine. Avoid confusion with "maple syrup urine" disease (9). Interactions with Diseases or Conditions: DIABETES: Fenugreek can alter blood sugar control in people with diabetes (15, 16). Blood glucose levels should be monitored closely. KIDNEY STONES (Nephrolithiasis): Theoretically, fenugreek can decrease calcium oxalate deposition and stone formation (4)
Dosage/Administration: ORAL: For diabetes, fenugreek 10 to 15 grams per day, as a single dose or in divided doses, with meals has been used (15, 16). A hydroalcoholic extract of fenugreek seeds 1 gram per day has also been used (21). For hyperlipidemia, 0.6 to 2.5 grams of fenugreek 2 times daily with meals has been used. It may be used alone or in combination with guar gum and other plant fibres (8, 16). For gastroesophageal reflux disease (GERD), a specific fenugreek product (FenuLife, Frutarom Belgium) 2 grams twice daily, 30 minutes before the two biggest meals of the day, has been used (23). Editor's Comments: The taste and odor of fenugreek resembles maple syrup, and it has been used to mask the taste of medicines (9). Fenugreek leaves are eaten in India as a vegetable (3)
Specific References: Fenugreek 1. Madar Z, Thorne R. Dietary fiber. Prog Food NutrSci 1987;11:153-74. 2. Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr 1990;44:301-6. 3. Patil SP, Niphadkar PV, Bapat MM. Allergy to fenugreek (Trigonella foenum graecum). Ann Allergy Asthma Immunol 1997;78:297-300. 4. Ahsan SK, Tariq M, Ageel AM, et al. Effect of Trigonella foenum-graecum and Ammi majus on calcium oxalate urolithiasis in rats. J Ethnopharmacol 1989;26:249-54. 5. Electronic Code of Federal Regulations. Title 21. Part 182 -- Substances Generally Recognized As Safe. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid= 78 6bafc6f6343634fbf79fcdca7061e1&rgn=div5&view= text&node=21:3.0.1.1.13&idno=21 6. Lambert J, Cormier J. Potential interaction between warfarin and boldo-fenugreek. Pharmacotherapy 2001;21:509-12.
| |
G |
|---|
GarlicGarlic
Aged Garlic Extract, Ail, Ajo, Allii Sativi Bulbus, Allium. Scientific Name: Allium sativum. Family: Alliaceae or Liliaceae.
Garlic is used for hypertension, hyperlipidemia, coronary heart disease, age-related vascular changes and atherosclerosis, myocardial infarction, earaches, chronic fatigue syndrome, and menstrual disorders. Garlic is also used to treat Helicobacter pylori infection. Other uses include treatment of allergic rhinitis, traveller's diarrhoea, colds, and flu. It is also used for immune system stimulation, and prevention and treatment of bacterial and fungal infections. Other uses include treatment of fever, coughs, headache, stomachache,sinus congestion, athlete's foot, gout, rheumatism, bronchitis, low blood pressure. It is also used as an aphrodisiac, for enhancing circulation, fighting stress and fatigue. Topically, garlic oil is used for fungal infections. Intravaginally, garlic is used alone or in combination with yogurt for vaginitis. In foods and beverages, fresh garlic, garlic powder, and garlic oil are used as flavour components.
Safety: No concerns regarding safety when used orally and appropriately. Garlic has been used safely in clinical studies lasting up to 7 years without reports of significant toxicity.63,64,65,66,67,68,69,70,71,72,73,74,75,76 Children: Likely to be safe when used orally and appropriately, short-term. In one study, garlic extract 300 mg three times daily had side effects comparable to placebo when used in children ages 8-18 years for eight weeks.77 There are no case reports available of significant adverse events or mortality in children associated with ingestion of garlic. Pregnancy and Lactation: No concerns regarding safety when used orally in amounts commonly found in foods.78 Effectiveness: POSSIBLY EFFECTIVE Atherosclerosis. Taking low doses of garlic powder orally, 300 mg per day, seems to lessen age-related decreases in aortic elasticity. Higher doses of 900 mg per day seem to slow development of atherosclerosis in both aortic and femoral arteries when used over a four-year period.79,72,73 Colorectal cancer. Several population studies suggest that increased dietary garlic consumption can decrease risk of developing colorectal cancer.84,80,81,82 However, garlic supplements do not seem to offer this benefit.83 Gastric cancer. Some evidence from population studies suggests that increasing dietary garlic consumption is associated with a decreased risk of developing stomach cancer.84,85,86 High Blood Pressure. Some clinical research shows that taking garlic orally can modestly reduce blood pressure in patients with hypertension and in people with normal blood pressure.87,88,89,63,75,90 In one analysis, garlic reduced systolic blood pressure by about 8% and diastolic blood pressure by about 7%, compared to placebo in patients with hypertension.90 Ringworm. Applying a garlic gel containing 0.6% ajoene seems to be as effective as terbinafine 1% cream.91 Tinea pedis (athlete's foot). Applying a garlic gel containing 1% ajoene seems to be more effective than 0.6% ajoene gel, and seems to beas effective as 1% terbinafine (Lamisil) for tinea pedis infections. Sixty days after completing one week of treatment 1% ajoene produces 100% mycologic cure, 0.6 % produces 72% mycologic cure, and 1% terbinafine produces 94% mycologic cure.92,93 INSUFFICIENT SCIENTIFIC EVIDENCE to RATE Common cold. Preliminary clinical research suggests garlic might reduce the frequency and number of colds when taken prophylactically.94
Mechanism of Action: The applicable part of garlic is the bulb. Garlic is mostly used for its antihyperlipidemic, antihypertensive, and antifungal effects. However, it is also reported to have antibacterial, antiparasite (worms), antiviral, antispasmodic, promotes sweating, expectorant, immunostimulant, and antithrombotic effects.95,96,97,98,99 Many of the pharmacological effects of garlic are attributed to the allicin, ajoene, and other organosulfur constituents such as S-allyl-L-cysteine.98
It's thought that the effectiveness of garlic products might to be determined by their ability to yield allicin, which in turn triggers production of other active constituents.100 Intact garlic cells in fresh garlic contain the odorless amino acid, alliin. When intact cells are broken, alliin comes intocontact with the enzyme alliinase in garlic, producing allicin, an unstable, odiferous compound.100,97 Fresh garlic contains approximately 1% alliin. One milligram of alliin is converted to 0.458mg allicin.101 Further conversion yields ajoene. The amount of allicin in garlic preparations is dependent upon the method of preparation. Processes that involve maceration of the garlic clove increase the activity of allicinase. Freeze-dried garlic may contain little or no allicin. To improve effectiveness, garlic preparations may be coated to protect the active constituents from degeneration by stomach acid.102 Heat and steam distillation used to produce garlic oil from crushed garlic converts allicin to allyl sulfides which are also thought to have biological activity.100
Garlic is aged to reduce the content of other sulfur compounds and the odor commonly associated with garlic. The process to produce odorless aged garlic extract reduces the alliin content to only 3% of what is typically contained in fresh garlic.101 Aged garlic extract is usually standardized to S-allyl-L-cysteine, another major organosulfur constituent in garlic.95
In patients with hyperlipidemia, garlic might lower cholesterol levels by acting as a HMG-CoA reductase inhibitor (statin).103,104 There is some evidence the constituent S-allyl-L-cysteine may be a potent inhibitor of hepatic cholesterol synthesis.105
For age-related vascular changes and atherosclerosis, garlic is thought to be beneficial and protect vascular endothelial cells from injury by reducing oxidative stress, inhibiting low-density lipoprotein (LDL) oxidation, and through antithrombotic effects.106,107,98,108 There is evidence that LDL oxidation may be inhibited by the constituents S-allyl cysteine, S-allyl mercaptocysteine, alliin, allixin, and by N-acetyl-S-allyl cysteine, a metabolite of S-allyl cysteine.108 Garlic appears to prevent endothelial cell depletion of glutathione, which may be responsible for its antioxidant effects.106
Garlic powder and aged garlic preparations have been shown to have antiplatelet properties in both patients with cardiovascular disease and in healthy volunteers.109,95,96,110,111 Garlic has been found to have antithrombotic properties and can increase fibrinolytic activity,decrease platelet aggregation and adhesion, increase the prothrombin time (PT), and inhibit metabolic enzymes in platelets responsible for the conversion of arachidonic acid into prostaglandins and other products.95,96,98,112 Raw garlic seems to have more potent antiplatelet properties than cooked garlic.113,114,115 Crushing garlic before cooking might prevent some of the loss of antiplatelet activity.115 Garlic oil does not appear to affect platelet aggregation.116
Garlic is thought to reduce blood pressure by causing smooth muscle relaxation and vasodilation by activating production of endothelium-derived relaxation factor (EDRF, nitric oxide).117
Garlic also seems to have humoral and cellular immunostimulant activity.
The constituents allicin and ajoene are thought to beresponsible for garlic's antifungal activity against ringworm infections.98,93 Fresh garlic, but not aged garlic, has shown activity against Escherichia coli, methicillin-resistant Staph aureus, salmonella enteritidis, and Candida albicans in the laboratory; it has been suggested as a food additive to prevent food poisoning. 118 Preliminary evidence suggests that garlic compounds might have activity against viruses
Adverse Reactions: Orally, garlic has dose-relatedadverse effects, which most commonly include breath and body odour, mouth and gastrointestinal burning or irritation, heartburn, flatulence, nausea, vomiting, and diarrhoea. These effects can be more pronounced with consumption of raw garlic or in patients unaccustomed to eating garlic.78,65,101 Oral use of garlic can also cause changes to the intestinalflora,78 ,101 which might result in gastrointestinal upset. Garlic's effect on platelet function is well known, and can possibly increase the risk of bleeding.
Interactions with Herbs & Supplements: Anticoagulatn/Antiplatelet Herbs and Supplements: Concomitant use of herbs that have constituents that might affect platelet aggregation could theoretically increase the risk of bleeding in some people. These herbs include angelica, clove, danshen, ginger, ginkgo, red clover, turmeric, vitamin E, willow, and others.109,95,96,110,111
Interactions with Drugs: Anticoagulatn/Antiplatelet Drugs including Warfarin. Cyclosporine: (transplant patients). Isoniazid (TB treatment). Saquinavir (HIV treatment).
Interactions with Foods: None known.
Interactions with Lab Tests: Blood Pressure: Garlic can lower blood pressure and blood pressure readings.87,88,89 Cholesterol: Garlic can lower serum cholesterol concentrations and test results.87,88,89 Clotting Studies.120
Interactions with Diseases or Conditions: Bleeding Disorders:95,96,98 Contraindicated. Gastrointestinal (GI) Irritation: Garlic can irritate the GI tract; dose related, resolves on stopping garlic. 78,65,101 Surgery: discontinue one to two weeks prior to scheduled surgery.121,101,122
Dosage/Administration: Dr Clare’s Blends: 1gm/day
Specific References: GARLIC 63. Steiner M, Khan AH, Holbert D, Lin RI. A double-blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am J Clin Nutr 1996;58:866-70. 64. Holzgartner H, Schmidt U, Kuhn U. Comparison of the efficacy and tolerance of a garlic preparation vs. bezafibrate. Arzneimittelforschung 1992;36:1473-7. 65. Jain AK, Vargas R, Gotzkowsky S, McMahon FG. Can garlic reduce levels of serum lipids? A controlled clinical study. Am J Med 1993;94:632-5. 66. Mader FH. Treatment of hyperlipidaemia with garlic-powder tablets. Evidence from the German Association of General Practitioners' multicentric placebo-controlled double-blind study. Arzneimittelforschung 1990;34:1111-6. 67. Rotzsch W, Richter V, Rassoul F, Walper A. [Postprandial lipemia under treatment with Allium sativum. Controlled double-blind study of subjects with reduced HDL2-cholesterol]. [Article in German]. Arzneimittelforschung 1992;36:1223-7. 68. Silagy C, Neil A. Garlic as a lipid lowering agent--a meta-analysis. J R Coll Physicians Lond 1994;28:33-39. 69. Vorberg G, Schneider B. Therapy with garlic: results of a placebo-controlled, double-blind study. Br J Clin Pract Symp Suppl 1990;69:7-11. 70. Adler AJ, Holub BJ. Effect of garlic and fish-oilsupplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am J Clin Nutr 1997;59:445-44. 71. Morcos NC. Modulation of lipid profile by fish oil and garlic combination. J Natl Med Assoc 1997;89:673-8. 72. Breithaupt-Grogler K, Ling M, Boudoulas H, Belz GG. Protective effect of chronic garlic intake on elastic properties of aorta in the elderly.Circulation 1997;96:2649-49. 73. Koscielny J, Klussendorf D, Latza R, et al. The antiatherosclerotic effect of Allium sativum. Atherosclerosis 1999;144:237-43. 74. Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia: a meta-analysis of randomized clinical trials. Ann Intern Med 2000;133:420-9. 75. Ackermann RT, Mulrow CD, Ramirez G, et al. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med 2001;161:813-18. 76. You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 2006;98:974-83. 77. McCrindle BW, Helden E, Conner WT. Garlic extract therapy in children with hypercholesterolemia. Arch Pediatr Adolesc Med 1998;152:108 9-94. 78. Bloch AS. Pushing the Envelope of Nutrition Support: Complementary Therapies. Nutrition 2000;16:236-9. 79. Siegel G, Klubendorf D. The anti-atherosclerotic effect of Allium sativum: Statistics re-evaluated. Atherosclerosis 2000;150:437-8. 80. Steinmetz KA, Kushi LH, Bostick RM, et al. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol 1994;139:1-15. 81. Witte JS, Longnecker MP, Bird CL, et al. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol 1996;144:1015-19. 82. Le Marchand L, Hankin JH, Wilkens LR, et al. Dietary fiber and colorectal cancer risk. Epidemiology 1997;8:658-59. 83. Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis 1996;17:477-84. 84. Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr 2000;72:1047-46. 85. You WC, Blot WJ, Chang YS, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst 1989; 81:162-4. 86. Takezaki T, Gao CM, Ding JH, et al. Comparative study of lifestyles of residents in high and low risk areas for gastric cancer in Jiangsu Province, China; with special reference to allium vegetables. J Epidemiol 1999;9:297-305. 87. Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. J Hypertension 1994;12:463-8. 88. McMahon FG, Vargas R. Can garlic lower blood pressure? A pilot study. Pharmacotherapy 1993;13:406-7. 89. Auer W, Eiber A, Hertkorn E, et al. Hypertension and hyperlipidaemia: garlic helps in mild cases. Br J Clin Pract Symp Suppl 1990;69:3-6. 90. Ried K, Frank OR, Stocks NP, et al. Effect of garlic on blood pressure: A systematic review and meta-analysis. BMC Cardiovasc Disord 2008;8:13. 91. Ledezma E, Lopez JC, Marin P, et al. Ajoene in the topical short-term treatment of tinea cruris and tinea corporis in humans. Randomized comparative study with terbinafine. Arzneimittelforschung 1999;43:544-7. 92. Ledezma E, DeSousa L, Jorquera A, et al. Efficacyof ajoene, an organosulphur derived from garlic, in the short-term therapy of tinea pedis. Mycoses 1996;33:393-5. 93. Ledezma E, Marcano K, Jorquera A. Efficacy of ajoene in the treatment of tinea pedis: A double-blind and comparative study with terbinafine. J Am Acad Dermatol 2000;37:829-32. 94. Josling P. Preventing the common cold with a garlic supplement: a double-blind, placebo-controlled survey. Adv Ther 2001;18:189-93. 95. Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J Nutr 2000;130:2662-5. 96. Steiner M, Li W. Aged garlic extract, a modulator of cardiovascular risk factors: a dose-finding study on the effects of AGE on platelet functions. J Nutr 2001;131:980S-4S. 97. Ankri S, Mirelman D. Antimicrobial properties ofallicin from garlic. Microbes Infect 1999;1:125-9. 98. M, Thomson M, Afzal M. Garlic and onions: their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids 2000;56:49-73. 99. Lamm DL, Riggs DR. The potential application ofallium sativum (garlic) for the treatment of bladder cancer. Urol Clin North Am 2000;21:157-56. 100. Zhang XH, Lowe D, Giles P, et al. Gender may affect the action of garlic oil on plasma cholesterol and glucose levels of normal subjects. J Nutr 2001;131:1471-8. 101. Blumenthal M, Goldberg A, Brinckmann J, eds. Herbal Medicine Expanded Commission E Monographs. Newton, MA: Integrative Medicine Communications, 2000. 102. Staba EJ, Lash L, Staba JE. A commentary on the effects of garlic extraction and formulation on product composition. J Nutr 2001;131:1118S-9S. 103. Gebhardt R, Beck H. Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte cultures. Lipids 1996;31:1269-76. 104. Qureshi AA, Din ZZ, Abuirmeileh N, et al. Suppression of avian hepatic lipid metabolism by solvent extracts of garlic: impact on serum lipids. J Nutr 1983;113:1746-49. 105. Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr 2001;131:989S-93S. 106. Ide N, Lau BH. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine 1999;6:125-31. 107. Dirsch VM, Kiemer AK, Wagner H, Vollmar AM. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis 1998;139:333-9. 108. Lau BH. Suppression of LDL oxidation by garlic. JNutr 2001;131:985S-8S. 109. Steiner M, Lin RS. Changes in platelet function and susceptibility of lipoproteins to oxidation associated with administration of aged garlic extract. J Cardiovasc Pharmacol 1998;31:904-8. 110. Kiesewetter H, Jung F, Jung EM, et al. Effect of garlic on platelet aggregation in patients with increased risk of juvenile ischaemic attack. Eur JClin Pharmacol 1993;39:333-6. 111. Legnani C, Frascaro M, Guazzaloca G, et al. Effects of a dried garlic preparation on fibrinolysis and platelet aggregation in healthy subjects. Arzneimittelforschung 1993;37:119-22. 112. Evans V. Herbs and the brain: friend or foe? The effects of ginkgo and garlic on warfarin use. J Neurosci Nurs 2000;32:229-32. 113. Chutani SK, Bordia A. The effect of fried versus raw garlic on fibrinolytic activity in man. Atherosclerosis 1981;32:417-21. 114. Ali M, Bordia T, Mustafa T. Effect of raw versus boiled aqueous extract of garlic and onion on platelet aggregation. Prostaglandins Leukot Essent Fatty Acids 1999;54:37-7. 115. Cavagnaro PF, Camargo A, Galmarini CR, Simon PW. Effect of cooking on garlic (Allium sativum l.) Antiplatelet activity and thiosulfinates content. J Agric Food Chem 2007;49:1280-8. 116. Morris J, Burke V, Mori TA, et al. Effects of garlic extract on platelet aggregation: a randomized placebo-controlled double-blind study. Clin Exp Pharmacol Physiol 1995;22:414-7. 117. Pedraza-Chaverri J, Tapia E, Medina-Campos ON, et al. Garlic prevents hypertension induced by chronic inhibition of nitric oxide synthesis. Life Sci 1998;56:71-7. 118. Sasaki J, Kita T, Ishita K, et al. Antibacterial activity of garlic powder against Escherichia coli O-157. J Nutr Sci Vitaminol (Tokyo) 1999;39:785-90. 119. Weber ND, Andersen DO, North JA, et al. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med 1992;52:417-17. 120. Sunter WH. Warfarin and garlic. Pharm J 1991;246:722. 121. Burnham BE. Garlic as a possible risk for postoperative bleeding. Plast Reconstr Surg 1995;95:213. 122. Carden SM, Good WV, Carden PA, Good RM. Garlic and the strabismus surgeon. Clin Experiment Ophthalmol 2002;30:303-4. | |
GentianGentian
Bitter Root, Bitterwort, Gall Weed, Geneciana, Gentianae Radix, Gentiane, Gentiane Acaule, Gentiane Jaune, Gentiane Pâle, Gentiane Sans Tige, Gentiane Sauvage, Grande Gentiane, Pale Gentian, Racine Amère, Stemless Gentian, Yellow Gentian, Wild Gentian. CAUTION: See separate listings for Canadian Hemp and Jimson Weed.
Scientific Name: Gentiana lutea; Gentiana acaulis, synonym Gentiana kochiana. Family: Gentianaceae.
People Use This For: Orally, gentian is used for digestive disorders, such as loss of appetite, fullness, flatulence, diarrhea, gastritis, heartburn, and vomiting. It is used orally for fever; hysteria; hypertension; and stimulating menstrual flow; and as an antispasmodic, anthelmintic, and antiseptic. Topically, gentian is used for treating wounds and cancer.In combination with European elder flower, verbena, cowslip flower, and sorrel, gentian is used orally for maintaining healthy sinuses and treating sinusitis. It is used in combination with other products for malaria. In foods and beverages, gentian is used as an ingredient. In manufacturing, gentian is used in cosmetics.
Safety: LIKELY SAFE ...when the root preparations are used in amounts commonly found in foods. Gentian root has Generally Recognized As Safe status (GRAS) for use in foods in the US (3). POSSIBLY SAFE ...when gentian root is used orally in a specific combination that contains gentian root, elderflower, verbena, cowslip flower, and sorrel (SinuComp, Sinupret) (1, 2). There is insufficient reliable information available about the safety of the topical use of gentian. PREGNANCY AND LACTATION: There is insufficient reliable information available about the safety of gentian in medicinal amounts during pregnancy and lactation; avoid using.
Effectiveness: POSSIBLY EFFECTIVE Sinusitis. Taking gentian orally in a specific combination product that also contains elderflower, verbena, cowslip flower, and sorrel (SinuComp, Sinupret) seems to help treat acute or chronic sinusitis Clinical studies have used Sinupret (1, 2). There is insufficient reliable information available about the effectiveness of gentian for its other uses.
Mechanism of Action: The applicable parts of gentian are the root and bark. The root is most commonly used. Gentian root contains triterpenoids, xanthones, and other constituents (5, 6). Preliminary research suggests gentian root has sedative effects. The xanthone gentiacaulein seems to have antidepressant activity, possibly through inhibition of monoamine oxidase (MAO)-A (6). Preliminary research suggests that gentian bark extracts might have MAO-B inhibitor effects (7). Gentian root has been used historically as an antihypertensive. Gentian root extracts seem to have vasorelaxant properties (8, 10). Preliminary research suggests that the xanthone constituents gentiacaulein and gentiakochianin may be responsible for vasodilation by an unknown mechanism (9).
Adverse Reactions: Orally, gentian root in combination with other herbs can cause gastrointestinal adverse effects and allergic skin reactions (1, 2).
Interactions with Herbs & Supplements: HERBS AND SUPPLEMENTS WITH HYPOTENSIVE EFFECTS: Gentian is thought to have hypotensive effects. Theoretically, combining gentian with other herbs and supplements with hypotensive effects might increase the risk of hypotension. Some of these herbs and supplements include andrographis, casein peptides, cat's claw, coenzyme Q-10, fish oil, L-arginine, lycium, stinging nettle, theanine, and others.
Interactions with Drugs: ANTIHYPERTENSIVE DRUGS <<interacts with>> GENTIAN Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Possible • Level of Evidence = D Theoretically, concurrent use might increase risk of hypotension with drugs that lower blood pressure (8, 10). These include captopril (Capoten), enalapril (Vasotec), losartan (Cozaar), valsartan (Diovan), diltiazem (Cardizem), Amlodipine (Norvasc), hydrochlorothiazide (HydroDiuril), furosemide (Lasix), and many others.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: HYPOTENSION: Theoretically, gentian use might worsen hypotension or interfere with drug therapy to increase blood pressure (8, 10). SURGERY: Gentian might affect blood pressure. Theoretically, gentian might interfere with blood pressure control during and after surgical procedures. Tell patients to discontinue gentian at least 2 weeks before elective surgical procedures.
Dosage/Administration: chronic ORAL: For acute or sinusitis, a specific combination product (SinuComp Phytopharmica) containing gentian root 12 mg and 36 mgeach of European elder flower, verbena, sorrel, and cowslip flower has been used three times daily (1, 2). TOPICAL: No typical dosage.
Editor's Comments: The highly toxic white hellebore (Veratrum album) can be misidentified as gentian and has caused accidental poisoning when used in home-made preparations (4). Gentian root is unrelated to the gentian violet dye (methylrosaniline chloride).
Specific References: Ginseng 1. Neubauer N, Marz RW. Placebo-controlled, randomized,double-blind, clincal trial with Sinupret sugar coated tablets on the basis of a therapy with antibiotics and decongestant nasal drops in acute sinusitis. 1994;1:177-81. 2. Marz RW, Ismail C, Popp MA. Action profile and efficacy of a herbal combination preparation for the treatment of sinusitis. Wien Med Wochenschr 1999;149:202-8. 3. Electronic Code of Federal Regulations. Title 21. Part 182 -- Substances Generally Recognized As Safe. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/textidx?c=ecfr&sid= 786bafc6f6343634fbf79fcdca7061e1&rgn=div5&view= text&node=21:3.0.1.1.13&idno=21 4. Zagler B, Zelger A, Salvatore C, et al. Dietary poisoning with Veratrum album--a report of two cases. Wien Klin Wochenschr 2005;117:106-8. 5. Toriumi Y, Kakuda R, Kikuchi M, et al. New triterpenoids from Gentiana lutea. Chem Pharm Bull (Tokyo) 2003;51:89-91. 6. Tomic M, Tovilovic G, Butorovic B, et al. Neuropharmacological evaluation of diethylether extract and xanthones of Gentiana kochiana. Pharmacol Biochem Behav 2005;81:535-42. 7. Haraguchi H, Tanaka Y, Kabbash A, et al. Monoamine oxidase inhibitors from Gentiana lutea. Phytochemistry 2004;65:2255-60. 8. Uncini Manganelli RE, Chericoni S, Baragatti B. Ethnopharmacobotany in Tuscany: plants used as antihypertensives. Fitoterapia 2000;71:S95-100. 9. Chericoni S, Testai L, Calderone V, et al. The xanthones gentiacaulein and gentiakochianin are responsible for the vasodilator action of the roots of 144 Gentiana kochiana. Planta Med 2003;69:770-2. 10. Baragatti B, Calderone V, Testai L, et al. Vasodilator activity of crude methanolic extract of Gentiana kokiana Perr. et Song. (Gentianaceae). J Ethnopharmacol 2002;79:369-72. (Gentianaceae). J Ethnopharmacol 2002;79:369-72. | |
Ginger
Scientific name: Zingiber officinale. Botanical Family: Zingiberaceae. Part used: The parts of ginger used medicinally are the rhizome (root-like stem) and root. Traditional use. Ginger is used for: motion sickness, morning sickness, colic, dyspepsia, flatulence, chemotherapy-induced nausea, rheumatoid arthritis, osteoarthritis, loss of appetite, nausea and vomiting following surgery, and migraine headaches. It is also used for upper respiratory tract infections, coughs, bronchitis, for the promotion of sweating, as a circulatory stimulant and for treating stomach-ache, diarrhea and nausea for any reason. Ginger is commonly used as a flavoring agent in foods and beverages.
Safety. There are no concerns regarding safety when used appropriately. Ginger has been safely used in several clinical trials.(1,2,3,4,5,6,7,8,9,10,11,12,13)
Pregnancy: There are no concerns regarding safety. Studies in pregnant women suggest that ginger can be used safely and effectively for morning sickness without harm to the fetus. As with any medication given during pregnancy, the potential benefit to risk must be weighed. See dose guidelines for use in pregnancy.
Breastfeeding: There are no problems with food levels of ginger in the diet. No scientific studies have been undertaken on therapeutic doses. All herbs and spices cross into breastmilk so only use therapeutic doses of ginger if there is a clinical indication that warrants therapy, weighing up relative risks and benefits of using the herb. Constituents Volatile oil; predominantly hydrocarbons including zingiberene, ar-curcumene and bisabolene. Pungent principles; a mixture of phenolic compounds with carbon side chains. These are referred to as gingerols, gingerdiols, gingerdiones, dihydrogingerdiones and shaogaols. The shaogoals are formed during the drying process and are twice as pungent as the gingerols. Hence the dried ginger is more pungent than the fresh herb. Constituents. Oleoresin; including gingerol homologues including derivatives with a methyl sidechain. Gingerglycolipids 6-gingesulphonic acid. Starch, proteins (amino acids including arginine) and fat. Vitamins; including niacin, and vitamin A. Minerals. Scientific evidence. Morning sickness. Taking ginger orally seems to reduce the severity of nausea and vomiting with morning sickness. Ginger seems to be more effective than placebo and comparable to vitamin B6. (1,4,10,14,15,16) Vertigo. Taking 1 gram of ginger orally seems to reduce symptoms of vertigo, including nausea.(9) Osteoarthritis. Severalstudies have shown evidence of symptom relief as effective as ibuprofen and diclofenac (NSAIDs). One study showed benefit after twelve weeks of treatment. (5,6,7,23,24,25) Muscle Soreness. One study showed reduction in muscle soreness in women. (26) Painful periods. Two recent clinical trials in women demonstrated that ginger for 3 days from the start of their menstrual period was as effective as mefenamic acid (Ponstan) and ibuprofen (Nurafen) and placebo in relieving period pain. (27,28) Migraine headache. Anecdotal evidence suggests that ginger might reduce the severity and duration of migraine headache.(9)
Mechanism of action. Active constituents of ginger include gingerol, gingerdione, shogaol, and sesquiterpene and monoterpene volatile oils.(17,18) The chemical constituents of ginger vary among fresh, semi-dry, and dry forms of ginger.(17) Ginger has a variety of pharmacological properties including; lowering fever, analgesia, cough inhibition, anti-inflammatory, sedative, antibiotic, weak antifungal, and other properties.(19,17)
The mechanism by which ginger reduces nausea and vomiting might be due to the 6-gingerol constituent.(20) Other ginger constituents such as 6-shogaol and galanolactone seem to act on serotonin (a chemical transmitter in the nervous system, especially in the brain and gut) receptors.(20) Galanolactone seems to act primarily on serotonin receptors in the small intestine. Ginger has been shown to possess free radical scavenging, antioxidant, inhibition of lipid peroxidation and that these properties might have contributed to the observed gastro-protective effects. Ginger is sometimes used for inflammatory conditions such as osteoarthritis and rheumatoid arthritis. Some researchers speculate that certain constituents of ginger might inhibit pro-inflammatory enzymes.(21) Compounds found in ginger are capable of inhibiting PGE-2 production and that the compounds may act at several sites.(29)
Adverse reactions. Ginger is usually well tolerated when used in usual doses.
Interactions with herbs and supplements. None reported.
Interactions with drugs. None reported. Interaction with warfarin is generally cautioned. However a study showed that in healthy subjects there was no alteration in the metabolism of warfarin.(30,31) Undertake weekly monitoring for one month as an additional precaution.
Interactions with foods. None known.
Interactions with laboratory tests. None known.
Dosage. Recommended dose: 1.5-6mls per day 1:5 tincture 70% alcohol. Or for a stronger 1:2 tincture 90% alcohol the dose is from 0.25-0.5ml per day. Decoction: range from tsps. per day. Powder/capsule: range from 0.75-3gms per day. Osteoarthritis Oral: For morning sickness, 250 mg ginger 4 times daily, or 500 mg twice daily, has been used. (1,4,12,18) A higher dose of 650 mg 3 times daily has also been used.(16) It may take 3-4 days of regular use to be effective. For motion sickness, 1 gram of dried powdered ginger root 30 minutes to 4 hours before travel has been used.(8,22)
References. 1. Fischer-Rasmussen W, Kjaer SK, Dahl C, Asping U. Ginger treatment of hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol 1991;38:19-24. 2. Phillips S, Ruggier R, Hutchinson SE. Zingiber officinale (ginger)-an antiemetic for day case surgery. Anaesthesia 1993;48:715-7. 3. Bone ME, Wilkinson DJ, Young JR, et al. Ginger root-a new antiemetic. The effect of ginger root on postoperative nausea and vomiting after major gynaecological surgery. Anaesthesia 1990;45:669-71. 4. Vutyavanich T, Kraisarin T, Ruangsri R. Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial. Obstet Gynecol 2001;97:577-82. 5. Bliddal H, Rosetzsky A, Schlichting P, et al. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis Cartilage 2000;8:9-12. 6. Altman RD, Marcussen KC. Effects of ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum 2001;44:2531-38. 7. Marcus DM, Suarez-Almazor ME. Is there a role for ginger in the treatment of osteoarthritis? Arthritis Rheum 2001;44:2461-2. 8. Grontved A, Brask T, Kambskard J, Hentzer E. Ginger root against seasickness: a controlled trial on the open sea. Acta Otolaryngol 1998;105:45-9. 9. Grontved A, Hentzer E. Vertigo-reducing effect of ginger root. A controlled clinical study. ORL J Otorhinolaryngol Relat Spec 1986;48:282-6. 10. Portnoi G, Chng LA, Karimi-Tabesh L, et al. Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Am J Obstet Gynecol 2003;189:19-7. 11. Wigler I, Grotto I, Caspi D, Yaron M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthritis Cartilage 2003;11:783-9. 12. Smith C, Crowther C, Wilson K et al. A randomized controlled trial of ginger to treat nausea and vomiting in pregnancy. Obstet Gynecol 2004;103:639-45. 13. Manusirivithaya S, Sripramote M, Tangjitgamol S, et al. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int J Gynecol Cancer 2004;14:1063-9. 14. Jewell D, Young G. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 2000;(2):CD000145. 15. Borrelli F, Capasso R, Aviello G, et al. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol 2005;105:849-56. 16. Chittumma P, Kaewkiattikun K, Wiriyasiriwach B. Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: a randomized double-blind controlled trial. J Med Assoc Thai 2007;90:15-20. 17. Suekawa M, Ishige A, Yuasa K, et al. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J Pharmacobiodyn 1984;7:836-48. 18. Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. J Med Assoc Thai 2007;90:1703-9. 19. Langner E, Greifenberg S, Gruenwald J. Ginger: history and use. Adv Ther 1998;15:25-44. 20. Lumb AB. Mechanism of antiemetic effect of ginger. Anaesthesia 1993;48:1118. 21. Thomson M, Al-Qattan KK, Al-Sawan SM, et al. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids 2002;67:475-8. 22. Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth 2000;84:367-71. 23. Wigler I, Grotto I, Caspi D, Yaron M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthritis Cartilage 2003;11:783-9
24. Haghighi M, Khalva A, Toliat T, Jallaei S. Comparing the effects of ginger (Zingiber officinale) extract and ibuprofen on patients with osteoarthritis. Arch Iran Med 2005;8:267-71.
25. Drozdov VN, Kim VA, Tkachenko EV, Varvanina GG. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J Alt Compl Med 2012;18:583-8. 26. Int J Prev Med. 2013 Apr;4(Suppl 1):S11-5. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. Mashhadi NS1, Ghiasvand R, Askari G, Feizi A, Hariri M, Darvishi L, Barani A, Taghiyar M, Shiranian A, Hajishafiee M. 27. Giti Ozgoli, Marjan Goli, and Fariborz Moattar. The Journal of Alternative and Complementary Medicine. February 2009, 15(2): 129-132. doi:10.1089/acm.2008.0311. Published in Volume: 15 Issue 2: February 23, 2009 28. Effect of Zingiber officinale R. rhizomes (ginger) on pain relief in primary dysmenorrhea: a placebo randomized trial Parvin Rahnama, Ali Montazeri, Hassan Fallah Huseini, Saeed Kianbakht and Mohsen Naseri. BMC Complementary and Alternative Medicine 2012, 12:92 29. Phytomedicine. 2007 Feb;14(2-3):123-8. Epub 2006 May 18. The effect of extracts from ginger rhizome on inflammatory mediator production. Lantz RC1, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. 30. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects Xuemin Jiang1, Kenneth M. Williams2, Winston S. Liauw2, Alaina J. Ammit1, Basil D. Roufogalis1, Colin C. Duke1, Richard O. Day2 andAndrew J. McLachlan. British Journal of Clinical Pharmacology Volume 59, Issue 4, pages 425–432, April 2005 31. Badreldin H. Ali,Gerald Blunden, Musbah O. Tanira, Abderrahim Nemmar. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food and Chemical Toxicology Vol. 46, Issue 2. February 2008, Pages 409–420 | |
Gotu KolaGotu Kola
Also Known As: Brahma-Buti, Brahma-Manduki, Brahmi, Centella, Centella Asiática, Centella Asiatique, Centellase, Divya, Hydrocotyle, Hydrocotyle Asiatique, Hydrocotyle Indien, Indischer Wassernabel, Idrocotyle, Indian Pennywort, Indian Water Navelwort, Ji Xue Cao, Khulakhudi, Luei Gong Gen, Luo De Da, Madecassol, Mandukaparni, Manduk Parani, Mandukig, Marsh Penny, TTFCA, Talepetrako, Thick-Leaved Pennywort, Tsubo-kusa, Tungchian, White Rot. CAUTION: See separate listings for Brahmi and Cola Nut.
Scientific Name: Centella asiatica, synonym Hydrocotyle asiatica; Centella coriacea. Family: Apiaceae/Umbelliferae.
People Use This For: Orally, gotu kola is used for reducing fatigue, anxiety, depression, improving memory and intelligence, Alzheimer's disease, venous insufficiency including varicose veins, wound healing, and increasing longevity. It is also used for the common cold and influenza, swine flu, sunstroke, tonsillitis, pleurisy, urinary tract infection (UTI), hepatitis, jaundice, abdominal pain,diarrhoea, indigestion, gastritis, peptic ulcer disease, dysentery, trauma, shingles,leprosy, cholera, syphilis, psychiatric disorders, epilepsy, asthma, anaemia, and diabetes. Gotu kola is also used for contraception, amenorrhea, elephantiasis, systemic lupus erythematosus (SLE), tuberculosis, memory loss, and as an aphrodisiac. Topically, gotu kola is used for wound healing and reducing scars. Parenterally, gotu kola is used for bladder lesions associated with schistosomiasis.
Safety: POSSIBLY SAFE ...when used topically and appropriately. Gotu kola has been used safely in a cream and ointment for up to 8weeks (27, 28). ...when used orally. Gotu kola has been used safely in trials lasting up to 12 months (7, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26). However, there is concern that gotu kola might cause hepatotoxicity in some patients (29). PREGNANCY: POSSIBLY SAFE ...when used topically and appropriately (28, 31). There is insufficient reliable information available about the safety gotu kola when used orally during pregnancy; avoid using. LACTATION: Insufficient reliable information available; avoid using.
Effectiveness: POSSIBLY EFFECTIVE Venous insufficiency. Taking gotu kola orally for 4-8 weeks seems to improve measures of circulation and decrease symptoms such as edema (7, 11, 18, 19, 21, 25). INSUFFICIENT RELIABLE EVIDENCE to RATE Atherosclerosis. There is preliminary evidence that taking gotu kola orally for 12 months might help stabilize low-density atherosclerotic plaques. Stabilizing these plaques might reduce the risk of rupture and embolism (17,24). Deep vein thrombosis (DVT). Gotu kola helps prevent thrombosis related to long-distance flights.Preliminary evidence suggests that gotu kola might decrease edema, and improve measures of circulation in patients traveling on airplanes for more than 3 hours (22); however, it is not known if t his actually results in a decreased incidence of clots. Diabetic microangiopathy. Taking gotu kola orally for 6-12 months might help increase measures of circulation, and decrease edema in patients with diabetic microangiopathy (20, 23). Keloids. There is some evidence that applying an extract of gotu kola, known as madecassol, topically might help reduce keloids and hypertrophic scarring (30). Psoriasis. Some evidence suggests that applying gotu kola topically might help reduce symptoms of psoriasis (6). Scarring: Preliminary evidence suggests that applying a specific gotu kola cream (Alpha centella, not available in the US) twice daily for 6-8weeks following suture removal might help reduce scarring in patients without a history of keloid formation (27). Schistosomiasis. There is some evidence that using gotu kola parenterally might help bladder lesions of schistosomiasis (bilharzial infections) (10). Stretch marks (striae gravidarum). Preliminary evidence suggests that applying a specific mixture of gotu kola, vitamin E, and collagen-elastin hydrolysates in a cream (Trofolastin, not available in the US) daily during the second and third trimesters of pregnancy until labor might reduce stretch marks (31). There is also preliminary evidence that another specific mixture of gotu kola, vitamin E, essential fatty acids, hyaluronic acid, elastin, and menthol in an ointment (Verum, not available in the US) might help decrease the formation of stretch marks during pregnancy (28). Wound healing. Preliminary evidence suggests that applying gotu kola topically might help improve wound healing (7). More evidence is needed to rate gotu kola for these uses.
Mechanism of Action: The applicable parts of gotu kola are the above groundparts. The primary constituents responsible for the pharmacological effects are thought to be the saponin-containing triterpene acids, 1% to 8%; and their sugar esters, including asiatic acid, madecassic acid, asiaticoside A (madecassoside), and asiaticoside B (26). Gotu kola also contains essential oils; flavonoids; and flavone derivatives including quercetin and kaempferol, sesquiterpenes, stigmasterol, sitosterol, and isothankuniside (713). In vitro evidence suggests that gotu kola might bind cholecystokinin (CCK) and GABA receptors, which might be responsible for reported anxiolytic effects of gotu kola (6, 26). The effects on GABA might also results in sedative, anticonvulsant, and analgesic effects (7). There is some evidence that taking gotu kola orally might to reduce the startle response in healthy volunteers (26). Some researchers think the asiaticoside derivatives, asiatic acid, asiaticoside 6, and SM2, might have a role in Alzheimer's disease. Preliminary evidence that they might protect neurons from beta-amyloid toxicity (3). The triterpenoid saponins (e.g., asiaticoside, madecassoside) seem to increase wound healing and decrease venous pressure in venous insufficiency (2. 5, 11). Asiaticoside and other terpenoids might have anti-inflammatory activity (27, 32). The terpenoid extract seems to improve connective tissue re modeling by increasing fibroblast activity, stimulating collagen synthesis, increasing epithelial turnover over, and decreasing capillary permeability (16,17,19). Gotu kola is thought to increase the production of type I collagen in scar formation over type II. Type II collagen is associated with hypertrophic scarring (27). The terpenoid extract might help stabilize arterial plaques by increasing collagen within plaques. Plaques with low collagen content are structurally weak and are associated with an increased risk of rupture and embolism (17, 24)). There is also some evidence that asiaticosides might promote wound healing by stimulating collagen and glycosaminoglycan synthesis (4, 5). There's preliminary evidence that asiaticosides might also have preventive and therapeutic effects on gastrointestinal ulcers (7, 8). Anti-ulcer mechanisms may be due to strengthening action on gastric mucosal lining and suppression of damaging effects of free radicals (8). Preliminary evidence suggests that purified isothankuniside might decrease fertility. However, a crude extract of gotu kola does not reduce fertility (13). Gotu kola extracts also seem to have antibacterial activity in vitro against Pseudomonas pyocyaneus, Trichoderma mentagrophytes, and Entamoeba histolytica. It also seems to have antiviral activity againstHerpes simplex type II (7). There is some interest in using gotu kola to treat cancer. Dried powder extracts of gotu kola exhibit cytotoxic and anti-tumor properties in preliminary studies. Normal lymphocytes are not harmed, which suggests gotu kola exerts selective toxicity towards tumor cells (9). There are some case reports of hepatotoxicity. The triterpenoids contained in gotu kola are theorized to be responsible for this adverseeffect (29).
Adverse Reactions: Orally, gotu kola is usually well-tolerated when used in typical doses (17, 18, 19, 20, 22, 23, 24, 25, 26). However, in some patients it can cause gastrointestinal upset and nausea (7). Theoretically, gotu kola might also cause drowsiness (7). There are at least three cases of hepatotoxicity associated with gotu kola. In one case, a 61-year-old woman developed elevated liver function tests (LFTs) including AST, ALT, alkaline phosphatase, and total bilirubin after taking gotu kola tablets for 30 days. Liver biopsy showed granulomatous acute hepatitis, suggesting an immune mediated hepatitis. LFTs improved when gotu kola was discontinued. Months later the patient took gotu kola again and developed elevated LFTs again after 2 weeks. In another case, a 52-year-old woman developed symptoms of hepatitis and increased LFTs after taking gotu kola for 3 weeks. Biopsy indicated chronic hepatitis and granulomas,areas of necrosis, and cirrhotic transformation. LFTs normalized after discontinuation of gotu kola. In a third case, a 49-year-old woman developed symptoms of hepatitis and elevated LFTs 2 months after starting gotu kola. Biopsy revealed granulomatous hepatitis. LFTs normalized after discontinuation of gotu kola (27). Researchers did not perform laboratory analysis of the products taken in these cases to determine if they were free of hepatotoxic contaminants. Therefore, it is not possible to rule out product contamination. The doses of gotu kola taken by these patients is unknown. Therefore, it is not known if higher doses are more likely than lower doses to cause hepatotoxicity. In a clinical trial where liver function was monitored, patients taking gotu kola 120 mg/day for 6 months, no changes in hepatic function were observed (20).
Topically, gotu kola can cause allergic contact dermatitis, characterized by erythema, itching, papules, and a burning sensation (1,7, 12). Gotu kola may also cause eczema when applied topically (14, 15).
Interactions with Herbs & Supplements: HEPATOTOXIC HERBS AND SUPPLEMENTS: There is some concern that gotu kola might cause hepatotoxicity in some patients (29). Theoretically, concomitant use with other potentially hepatotoxic products might increase the risk of developing liver damage. Some of these products include androstenedione, chaparral, comfrey, DHEA, germander, niacin, pennyroyaloil, red yeast, and others. HERBS AND SUPPLEMENTS WITH SEDATIVE PROPERTIES: Theoretically, concomitant use with herbs that have sedative properties might enhance therapeutic and adverse effects (7). Some of these supplements include 5-HTP, calamus, California poppy, catnip, hops, Jamaican dogwood, kava, St. John's wort, skullcap, valerian, yerba mansa, and others.
Interactions with Drugs: Topically, gotu kola can cause allergic contact dermatitis, characterized by erythema, itching, papules, and a burning sensation (1,7, 12). Gotu kola may also cause eczema when applied topically (14, 15).
Interactions with Herbs & Supplements: HEPATOTOXIC HERBS AND SUPPLEMENTS: There is some concern that gotu kola might cause hepatotoxicity in some patients (29). Theoretically, concomitant use with other potentially hepatotoxic products might increase the risk of developing liver damage. Some of these products include androstenedione, chaparral, comfrey, DHEA, germander, niacin, pennyroyal oil, red yeast, and others. HERBS AND SUPPLEMENTS WITH SEDATIVE PROPERTIES: Theoretically, concomitant use with herbs that have sedative properties might enhance therapeutic and adverse effects (7). Some of these supplements include 5-HTP, calamus, California poppy, catnip, hops, Jamaican dogwood,kava, St. John's wort, skullcap, valerian, yerba mansa, and others.
Interactions with Drugs: CNS DEPRESSANTS <<interacts with>> GOTU KOLA Interaction Rating = Major Do not take this combination. Severity = High • Occurrence = Probable • Level of Evidence = D Theoretically, concomitant use of gotu kola with drugs with sedative properties might cause additive effects and side effects (7). Some sedative drugs include clonazepam (Klonopin), lorazepam (Ativan), phenobarbital (Donnatal), zolpidem (Ambien), and others. HEPATOTOXIC DRUGS <<interacts with>> GOTU KOLA Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = D There is some concern that gotu kola might cause hepatotoxicity in some patients (29). Theoretically, concomitant use with other potentially hepatotoxic drugs might increase the risk of developing liver damage. Some of these drugs include acarbose (Precose, Prandase), amiodarone (Cordarone), atorvastatin (Lipitor), azathioprine (Imuran), carbamazepine (Tegretol), cerivastatin (Baycol), diclofenac (Voltaren), felbamate (Felbatol), fenofibrate (Tricor), fluvastatin (Lescol), gemfibrozil (Lopid), isoniazid, itraconazole, (Sporanox), ketoconazole (Nizoral), leflunomide (Arava), lovastatin (Mevacor), methotrexate (Rheumatrex), nevirapine (Viramune), niacin, nitrofurantoin (Macrodantin), pioglitazone (Actos), pravastatin (Pravachol), pyrazinamide, rifampin (Rifadin), ritonavir (Norvir), rosiglitazone (Avandia), simvastatin (Zocor), tacrine (Cognex), tamoxifen, terbinafine (Lamisil), valproic acid, and zileuton (Zyflo).
Interactions with Foods: None known. Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: LIVER DISEASE: There is concern that gotu kola might be linked to cases of hepatotoxicity (29). Theoretically, gotu kola might exacerbate liver problems in patients with existing liver disease such as hepatitis. Advise these patients to avoid taking gotu kola. SURGERY: Gotu kola has CNS depressant effects. Theoretically, gotu kola might cause additive CNS depression when combined with anesthesia and other medications during and after surgical procedures. Tell patients to discontinue gotu kola at least 2 weeks before elective surgical procedures. Dosage/Administration: ORAL: For atherosclerosis, 60 mg of gotu kola extract three times daily has been used (17, 24). For diabetic microangiopathy, 60 mg of gotu kola extract twice daily has been used (20, 23). For venous insufficiency, 60-180 mg daily of gotu kola extract has been used (18, 19, 21, 25). For deep vein thrombosis (DVT) prevention while flying, 120 mg of gotu kola extract 3 days before the flight, the day of the flight, and the day after have been used (22). TOPICAL: For wound healing, 1% gotu kola creams have been used (7). For scars, gotu kola has been used in combination with the extract from Bulbine frutescens (Alpha centella cream, not available in the US) for 6-8weeks (27). For preventing stretch marks in pregnancy, gotu kola has been used in combination with several other ingredients (Trofolastin and Verum). Trofolastin cream (not available in the US) is a mixture of gotu kola, vitamin E, and collagen-elastin hydrolysates (21). Verum ointment (not availablein the US) contains gotu kola, vitamin E, essential fatty acids, hyaluronic acid, elastin, and menthol (28).
Editor's Comments: Gotu kola is a commonly used herb in Tradition Chinese and Ayurvedic medicine (26). The terpenoid extract of gotu kola is sometimes referredto as TTFCA (total terpenoid fraction of Centella asiatica) and Centellase. Avoid confusion with cola nut (Cola acuminata), and swamp pennywort (Centella cordifolia). Centella cordifolia is often misidentified as Centella asiatica (gotu kola).
Specific References: 1. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 2. Pointel JP, Boccalon H, Cloarec M, et al. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiol 161 1987;38:46-50. 3. Mook-Jung I, Shin JE, Yun SH, et al. Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. J Neurosci Res 1999;58:417-25. 4. Maquart FX, Chastang F, Simeon A, et al. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol 1999;9:289-96. 5. Shukla A, Rasik AM, Jain GK, et al. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol 1999;65:1-11. 6. Kenady DE, Chretien PB, Potvin C, Simon RM. Thymosin reconstitution of T-cell deficits in vitro in cancer patients. Cancer 1977;39:575-80. 7. Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the east Asian medical plant Centella asiatica. Phytomedicine 2000;7:427-48. 8. Cheng CL, Koo MWL. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci 2000;67:2647-53. 9. Babu TD, Kuttan G, Padikkala J. Cytotoxic and anti-tumour properties of certain taxa of Umbelliferae with special reference toCentella asiatica (L.) Urban. J Ethnopharmacol 1995;48:53-7. 10. Fam A. Use of titrated extract of Centella asiatica (TECA) in bilharzial bladder lesions. Int Surg 1973;58:451-2. 11. Belcaro GV, Rulo A, Grimaldi R. Capillary filtration and ankle edema in patients with venous hypertension treated with TTFCA. Angiology 1990;41:12-8. 12. Eun HC, Lee AY. Contact dermatitis due to madecassol. Contact Dermatitis 1985;13:310-3. 13. Dutta T, Basu UP. Crude extract of Centella asiatica and products derived from its glycosides as oral antifertility agents. Indian J Exp Biol 1968;6:181-2. 14. Hausen BM. Centella asiatica (Indian pennywort), an effective therapeutic but a weak sensitizer. Contact Dermatitis 1993;29:175-9. 15. Bilbao I, Aguirre A, Zabala R, et al. Allergic contact dermatitis from butoxyethyl nicotinic acid and Centella asiatica extract.Contact Dermatitis 1995;33:435-6. 16. Incandela L, Cesarone MR, Cacchio M, et al. Total triterpenic fraction of Centella asiatica in chronic venous insufficiency and in high-perfusion microangiopathy. Angiology 2001;52 Suppl 2:S913. 17. Incandela L, Belcaro G, Nicolaides AN, et al. Modification of the echogenicity of femoral plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, randomized, placebo-controlled trial. Angiology 2001;52 Suppl 2:S69-73. 18. Incandela L, Belcaro G, De Sanctis MT, et al. Total triterpenic fraction of Centella asiatica in the treatment of venous hypertension: a clinical, prospective, randomized trial using a combined microcirculatory model. Angiology 2001;52 Suppl 2:S61-7. 19. De Sanctis MT, Belcaro G, Incandela L, et al. Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology 2001;52 Suppl 2:S55-9 20. Cesarone MR, Incandela L, De Sanctis MT, et al. Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centella asiatica: a clinical prospective randomized trial with a microcirculatory model. Angiology 2001;52 Suppl 2:S49-54. 21. Cesarone MR, Belcaro G, Rulo A, et al. Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: measurement by laser Doppler, TcPO2-CO2, and leg volumetry. Angiology 163 2001;52 Suppl 2:S45-8. 22. Cesarone MR, Incandela L, De Sanctis MT, et al. Flight microangiopathy in medium- to long distance flights: prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology 2001;52 Suppl 2:S33-7. 23. Incandela L, Belcaro G, Cesarone MR, et al. Treatment of diabetic microangiopathy and edema with total triterpenic fraction of Centella asiatica: a prospective, placebo-controlled randomized study. Angiology 2001;52 Suppl 2:S27-31. 24. Cesarone MR, Belcaro G, Nicolaides AN, et al. Increase in echogenicity of echolucent carotid plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, placebo-controlled, randomized trial. Angiology 2001;52 Suppl 2:S19-25. 25. Cesarone MR, Belcaro G, De Sanctis MT, et al. Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Angiology 2001;52 Suppl 2:S15-18. 26. Bradwejn J, Zhou Y, Koszycki D, Shlik J. A double-blind, placebo-controlled study on the effects of Gotu Kola (Centella asiatica) on acoustic startle response in healthy subjects. J Clin Psychopharmacol 2000;20:680-4. 27. Widgerow AD, Chait LA, Stals R, Stals PJ. New innovations in scar management. Aesthetic Plast Surg 2000;24:227-34. 28. Young GL, Jewell D. Creams for preventing stretch marks in pregnancy. Cochrane Database Syst Rev 2000;(2):CD000066. 29. Jorge OA, Jorge AD. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev Esp Enferm Dig 2005;97:115-24. 30. Bosse JP, Papillon J, Frenette G, et al. Clinical study of a new antikeloid agent. Ann Plast Surg 1979;3:13,21. 31. Mallol J, Belda MA, Costa D, et al. Prophylaxis of striae gravidarum with a topical formulation. A double blind trial. Int J Cosmet Sci 1991;3:51-7. 32. Guo JS, Cheng CL, Koo MW. Inhibitory effects of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats. Planta Med 2004;70:1150-4.
| |
Green tea
Scientific name: Camellia sinensis, it is from the exact same tea plant as Black tea commonly used throughout the world. Botanical family: Theaceae. Parts used: Bud leaf and stem.
Traditional use. Green tea has been consumed and enjoyed as a beverage for centuries. Green tea is produced by steaming fresh leaves at high temperature. Unlike black and oolong (partially fermented) teas, green tea is not fermented. Improving cognitive performance and mental alertness is a traditional use for green tea. It is also used to treat stomach disorders, vomiting, diarrhea, and headaches. Green tea has a history of use for weight loss. It has a role in cancer prevention and treatment including breast cancer, cervical cancer, prostate cancer, colon cancer, gastric cancer, lung cancer, leukemia, and skin cancer related to ultraviolet radiation (e.g. sunburn) and other environmental causes. It has traditionally been used for its anti-viral effects on the wart virus including genital warts, perianal warts and cervical cell changes. Other uses include osteoporosis, Crohn's disease, Parkinson's disease, cardiovascular disease, diabetes, low blood pressure, chronic fatigue syndrome, tooth decay, kidney stones, and skin damage.
Topically, green tea is used as a wash to soothe sunburn, as a poultice for bags under the eyes, as a compress for headache or tired eyes, and to stop the bleeding of tooth sockets. Green tea in chewable candy is used for inflammation of the gums. Green tea is also used topically to prevent skin damage and cancer related to ultraviolet radiation (e.g. sunburn) and other environmental causes.
Safety. There are no concerns about safety when consumed as a beverage in moderate amounts of 1-4 cups of green tea per day. (1,2,3,4,5,6,7,8) Green tea extract as a supplement containing 7% caffeine has been used safely for six months. (9) Avoid using high doses long-term because Green tea contains a significant amount of caffeine. Typically a cup of green tea contains 35-60mgs of caffeine. Children: While no safety concerns have been highlighted avoid caffeinated drinks in children and limit to 1 cup per day. (11,12) Avoid giving green tea to infants, as they have an increased risk of anemia. (88) Pregnancy: There are no reported problems when used orally in moderate amounts of two cups per day. Breastfeeding: There are no particular concerns when used orally in moderate amounts of 1-4 cups per day. If the baby has loose stools exclude green tea, black tea and coffee to see if caffeine is contributing to the problem. Constituents.
Scientific Evidence. Mental Alertness. Green tea and other caffeinated beverages prevent a decline in alertness and cognitive capacity when consumed throughout the day. (10,13,14)
Bladder cancer, esophageal cancer, and pancreatic cancer. cervical dysplasia. Drinking green tea is associated with a reduced the risk of bladder cancer, esophageal cancer, and pancreatic cancer. (15,16,17,18,19) Green tea as an oral or topical preparation seems to reduce cervical dysplasia caused by human warts (papilloma virus) infection. (20).
High Cholesterol. Green tea taken orally lowers cholesterol and triglycerides.(21)(22) Low Blood Pressure. Consuming caffeinated beverages including Green Tea increases blood pressure in elderly people with low blood pressure after meals which may prevent falls. (23,24) Oral leukoplakia (lesions of the mouth that may progress to cancer). Drinking green tea orally decreases the size of lesions in patients with oral leukoplakia. (25) Ovarian cancer; Women who regularly consume green tea or black tea, have a lower risk of developing ovarian cancer.(92,25,26,27) Parkinson's disease. Consuming green tea orally helps prevent or delay the onset of Parkinson's disease. (28, 29) Prevention of colorectal cancer. Population studies suggest that consuming green tea does not have any effect on colon cancer risk. (30,31,32)
Breast cancer. Population studies suggest that green tea does not seem to reduce the risk of initially developing breast cancer in Asian populations; (33.34) however, in Asian-American populations some evidence suggests that drinking green tea might reduce the risk of developing breast cancer. (35) Additional population research suggests that Asian women who have had early breast cancer (Stage 1-11) who drink 3-5 of more cups of green tea daily seem to have reduced risk of breast cancer recurrence. (36,33) Cardiovascular disease. A large-scale population study in Japan suggests that consuming 3 or more cups of green tea daily significantly decreases the risk of cardiovascular and all-cause mortality compared to drinking less than one cup daily. (32) Diabetes. Population studies suggests that Japanese adults who consume 6 or more cups/day of green tea have a 33% lower risk of developing type 2 diabetes.(37) Gastric Cancer. There is conflicting evidence about the effects of green tea on gastric cancer risk. Gingivitis (inflammation of the gums); Green tea extract in chewable candy appears to reduce inflammation. (38) High Blood Pressure. There is evidence of variable effects of green tea depending on the physiological state of the tissues when taken by individuals. Population studies in Chinese people show that drinking 120-599 mL of green tea or oolong tea daily is associated with a 46% lowered risk of developing hypertension compared with non-habitual tea drinkers. Drinking more than 600 mL per day is associated with a 65% reduced risk. (39) However, clinical studies on people with normal and high blood pressure show that green tea or black tea has no effect on their blood pressure. (40) This apparent contradiction is common with herbal medicines who only have an action when it is required to normalise the physiological terrain. It is one of the benefits of multi constituent medicines. Active constituents can vary from person to person depending on their individual physiology. Lung Cancer. There is conflicting evidence about the effects of green tea on lung cancer risk. (32) (41) Obesity. There is conflicting evidence about the effectiveness of green tea for obesity and weight loss. A meta-analysis of clinical studies suggests that, overall, taking green tea extract 576-714 mg/day along with caffeine seems to modestly reduce body mass index (BMI), body weight, and waist circumference compared to caffeine alone. But taking green tea extract without caffeine does not seem to significantly reduce weight or waist circumference. (42) (43) Osteoporosis. Population research suggests that drinking green tea for ten years is associated with increased bone mineral density. (44) Prostate cancer. Chinese men who consume green tea seem to have a lower risk of developing prostate cancer. (45) Preliminary clinical research suggests that men with advanced cancer who take green tea supplements (containing catechins 200 mg three times daily) for a year seem to have a reduced risk of progression to prostate cancer. (46) Osteoarthritis
Mechanism of action. Polyphenols including flavanols, flavandiols, flavonoids, and phenolic acids are abundant in green tea. The flavanols are all referred to as catechins. These seem to be responsible for many of the proposed benefits of green tea. (19,20,48,49,50) and they may be responsible for its anti-inflammatory activity. (51) They may inhibit the production of other inflammatory substances including COX-1and 2, leukotriene-B4 and the activity of 5-lipoxygenase and nitric oxide synthase.(52, 53) Green tea catechins may also protect cartilage by inhibiting proteoglycan and collagen breakdown.(54) Green tea polyphenols seem to lessen joint degeneration in laboratory models of rheumatoid arthritis. (55) Green tea also contains plant estrogens including beta-sitosterol. (41)
Green tea contains 2% to 4% caffeine or 10-80 mg caffeine per cup.(56) a typical amount is 35-60mgs per cup. The caffeine in green tea stimulates the central nervous system (CNS), heart, muscles, and possibly blood pressure.(57) Caffeine is thought to increase the release of neurotransmitters such as dopamine.(58) Caffeine also decreases airway resistance and stimulates respiration.(59) Caffeine may decrease GABA and Serotonin neurotransmitters in the Central Nervous System. (58) Caffeine stimulates stomach acid secretion, and increases fight or flight chemicals in the body.(60) Caffeine can have positive stimulating effects on the heart.(59) Caffeine can also immediately raise blood pressure, but might not have this effect in habitual users. (57) Caffeine doesn't substantially affect the fluid status of people who drink caffeinated beverages on a regular basis. (61,62) The caffeine content is also thought to be responsible for green tea's effects on mental performance. (13) Some preliminary studies show that flavonoids found in green tea might reduce heart disease risk factors lipoprotein oxidation. However, green tea doesn't reduce inflammation, vascular reactivity, or lipid oxidation. (63,64,65,66,67) Caffeine has been reported to cause increases and decreases in blood glucose. Green tea may protect against some kinds of cancer. Green tea polyphenols also appear to have activity against human wart virus and related cervical changes and genital warts (20,68) but the mechanism of action for this is not known. The polyphenols in green tea appear to reduce the cellular adhesiveness of bacteria associated with dental disease. (38) Some evidence suggests that green tea might be useful in skin disorders such as excess hair, acne and also male pattern baldness. (70) Green tea is thought to be beneficial for preventing skin damage and cancer from ultraviolet radiation. Areas of skin where green tea extracts were applied had fewer sunburned cells and less damage to skin cells. (71) Green tea is also used for weight loss. Early evidence indicates that a green tea extract rich in catechins can increase calorie and fat metabolism. The caffeine, catechin, and theanine constituents of green tea might contribute to this effect. (72,73,74) Caffeine increases resting energy expenditure and cellular heat production. (75) Tannins in green tea can reduce diarrhea and the polyphenols in green tea might have a beneficial effect on the gut flora.. (76) For prevention of Parkinson's disease, caffeine in green tea may help to maintain levels of dopamine in the central nervous system by preventing the inhibition of dopaminergic transmission. These actions help reduce the expression of symptoms of Parkinson’s disease. (28) Protecting people from developing Alzheimer's disease has got to be a major priority for aging Western populations. Early studies suggests that catechins in Green tea may prevent oxidation and cell death of neurons, which may help resist cell damage and maintain health. (77) Population studies suggest that drinking green tea for at least ten years increases bone mineral density. The exact mechanism for the effects on bone is unknown, but several possibilities have been suggested. Tea leaves contain fluoride, which might slow osteoporosis. Tea also contains flavonoids and phytoestrogens, which might affect bone mineral density. Other proposed mechanisms include inhibition of bone resorption and effects on mineral metabolism by polyphenols and tannins. (44)
Adverse reactions.
Green tea can cause digestive upset and dizziness, insomnia and agitation and confusion. These effects are more common with higher doses of green tea or green tea extract, equivalent to 5-6 liters of tea per day.(9,78) There have been at least 14 cases of liver toxicity, usually linked to green tea extract products in pill form. (79,80) However, there has been at least one report associated with consumption of a green tea-containing beverage. (80) In most reported cases, green tea products were not assessed to determine if any contaminants were present. In most cases, liver function returned to normal after discontinuation of the green tea product. (79,80)
Interaction with herbs and supplements.
Anticoagulant/antiplatelet herbs and supplements: There have been no reported cases of interaction with warfarin (this a pharmaceutical blood thinning agent). Caffeine containing herbs and supplements: Other natural products that contain caffeine include coffee, black tea, oolong tea, guarana, mate, cola, and others. Some supplements for energy may contain caffeine; check the label. Avoid large amounts of green tea along with supplements aimed at the market for sportspeople that contain creatine, caffeine or ephedra.(83,84) Liver toxic herbs and supplements: Theoretically, avoid taking green tea supplements with other hepatotoxic herbs or supplements as there might be additive effects. (79,85) Check out herbs you want to use with Green Tea and avoid the combination if there are concerns about liver function. Iron: Like black tea, green tea appears to reduce absorption of iron from foods. (86,87) However, a study of iron-deficient elderly patients suggests that concomitant use doesn't alter iron absorption in this population. (89) Iron levels are not affected in people with adequate iron intake. Theoretically, green tea might reduce the absorption of iron supplements. For most patients, this effect will not be clinically significant. On this account patients with iron deficiency are advised to consume black tea and green tea between meals. (87)
Interactions with drugs. There is preliminary evidence that green tea might enhance the effects of doxorubicin (Adriamycin) on cancer cells. (69) Caffeine accounts for most of the side effects for green tea. If you are on medication or need to start medication read the drug insert leaflet carefully. If there is an interaction with caffeine, or black tea or coffee be aware that green tea may have the same interaction.
Stimulants. Read the drug leaflet any nervous system stimulation medicine including amphetamines.
Drugs that inhibit caffeine metabolism; These include cimetidine (reduces stomach acid), fluconazole (anti-fungal), mexilitene (prescribed for heartbeat irregularities), fluvoxamine (antidepressant). Avoid green tea supplements or consult a medical herbalist. Drugs that decrease caffeine clearance: terbinafine (prescribed for fungal infection of toe nails), quinolone antibiotics (the commonest prescribed is ciproflaxacin, apart from nalidixic acid all others end with –acin). Hepatotoxic drugs. Avoid of green tea supplements. Estrogens. The effect of caffeine on estrogens is complicated and depends on race and the dose of caffeine. High caffeine levels can elevate estrogens and low doses can stimulate. An average amount in daily intake of green tea is unlikely to be clinically significant but may be relevant to supplement doses.93 Drugs that reduce caffeine clearance; theophylline. Warfarin. No reports have been noted with normal consumption, observe the usual vigilance. Interactions with foods. Avoid excess caffeine intake when used in conjunction with black tea and coffee. Because the effects of caffeine are dose related avoid excessive amount of caffeine intake. The amount of caffeine in coffee is extremely variable, check the packaging or server, black tea averages 30-80mgs per cup. Check the amount in soft drinks and avoid high caffeine energy drinks. Green tea appears to reduce absorption of iron from non-meat food sources. If you are concerned about this drink green tea between meals. Adding milk to your green tea may reduce the benefits of green tea as the milk may reduce the absorption of some of the helpful constituents. Interaction with laboratory tests. Inform your medical provider if you are taking green tea supplements. Drinking 4-6 cups of green tea per day is unlikely to affect laboratory tests any more than 1-3 cups of coffee. Interactions with diseases and conditions. Generalized anxiety or nervous excitability: avoid caffeinated beverages or supplements. Diabetes: caffeine may affect the presentation of hypoglycemic attacks. Avoid sudden increases or decreases of caffeine intake and monitor blood sugars more carefully if changing the amount of caffeine in your diet. Glaucoma: Intake of caffeinated beverage (>/=180 mg caffeine) may not be recommended for patients with normotensive glaucoma or ocular hypertension.
Bleeding time: Caffeine is reported to have antiplatelet activity however, this interaction has not been reported in humans. Heart rhythm irregularities: Caffeine in green tea can induce cardiac arrhythmias in sensitive individuals use with caution.
Dosage. It is difficult to be precise with dosage of green tea as much depends on the duration of brewing and the quality of the raw herb. Infusion: 1-6 cups for men per day and 1-4 cups for women per day if using one tsp. of the herb per cup of boiling water. Powder/capsule: 1500 mg per day. Ostearthritis Oral: Doses of green tea vary significantly, but usually range between 1-10 cups daily. The commonly used dose of green tea is based on the amount typically consumed in Asian countries, which is about 3 cups per day, providing 240-320 mg of polyphenols. The typical dose of caffeine:
References.
Jpn J Cancer Res 1988;79:1067-74.
| ||
H |
|---|
HawthornHawthorn
Crataegus, English Hawthorn, Whitethorn, Maybush, Maythorn.
Scientific Name: Crataegus monogyna; Crataegus laevigata, Crataegus oxyacantha. Family: Rosaceae.
People Use This For: Hawthorn is used for cardiovascular conditions such as congestive heart failure, coronary circulation problems, angina, and arrhythmias. It is also used to increase cardiac output reduced by hypertension or pulmonary disease, to treat both hypotension and hypertension, atherosclerosis. Hawthorn is also used as a sedative, anxiolytic, antispasmodic, astringent, and diuretic. In manufacturing, hawthorn is used for making candied fruit slices, jam, jelly, and wine.
Safety: No concerns regarding safety when used orally and appropriately, short-term. Hawthorn preparations seem to be safe when used for up to 16 weeks.21,22,23,24,25 Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: POSSIBLY INEFFECTIVE Congestive Heart Failure (CHF). There is contradictory evidence about the effects of hawthorn extract in heart failure patients. INSUFFICIENT RELIABLE EVIDENCE to RATE Anxiety. Preliminary clinical research suggests hawthorn, combined with magnesium and California poppy (Sympathyl, not available in the US), might be useful in treating mild to moderate anxiety More evidence is needed to rate hawthorn for this use.
Mechanism of Action: The applicable parts of hawthorn are the leaf, fruit, and flower. The constituents responsible for the pharmacological effects of hawthorn preparations include flavonoids and proanthocyanidins. Hawthorn preparations act on the myocardium by increasing force of contraction and lengthening the refractory period, increasing coronary blood flow and cardiac output, and reducing oxygen consumption.24,27,28 Hawthorn's cardiotrophic properties are attributed to increased membrane permeability for calcium, 27 and phosphodiesterase inhibition, which increases cell energy efficiency leading to increased coronary blood flow, vasodilation, and positive effects on heart muscle contraction.27,28 Preliminary research suggests that hawthorn also normalizes the heart rate.28 Hawthorn also seems to have blood pressure lowering activity, according to preliminary research. It seems to cause arterial relaxation. The proantocyanidin constituents seem to cause this effect. 28
Adverse Reactions: Hawthorn is generally well tolerated.24 Hawthorn preparations can uncommonly cause nausea, gastrointestinal complaints. Other side effects are rare.
Interactions with Herbs & Supplements: May have additive effects with other herbs in reducing Blood Pressure. Refer to a Medical Herbalist.
Interactions with Drugs: May have additive effects with drugs in reducing Blood Pressure. Refer to a Medical Herbalist.
Interactions with Foods: None known.
Interactions with Lab Tests: Cholesterol: Theoretically, hawthorn might lower blood levels total and low density lipoprotein (LDL) cholesterol and test results.28 Interactions with Diseases or Conditions: Heart Failure: Refer to a Medical Herbalist.
Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture. 400mgs-1gm daily.R2 pp.210
Specific References: HAWTHORN 21. Schmidt U, Kuhn U, Ploch M, Hubner WD. Efficacy of the Hawthorne (Crataegus) Preparation LI 132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine 1994;1:17-24. 22. Zapfe jun G. Clinical efficacy of crataegus extract WS 1442 in congestive heart failure NYHA class II. Phytomedicine 2001;8:262-6. 23. Tauchert M. Efficacy and safety of crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am Heart J 2002;143:910-5. 24. Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med 2003;114:665-74. 25. Holubarsch CJ, Colucci WS, Meinertz T, et al. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur J Heart Fail 2008;10:1255-63. 26. Hanus M, Lafon J, Mathieu M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium inmild-to-moderate anxiety disorders. Curr Med Res Opin 2004;20:63-71. 27. Schwinger RH, Pietsch M, Frank K, Brixius K. Crataegus special extract WS 1442 increases force of contraction in human myocardium cAMP-independently. J Cardiovasc Pharmacol 2000;35:700-7. 28. Chang Q, Zuo Z, Harrison F, Chow MS. Hawthorn. J Clin Pharmacol 2002;42:605-12. | |
HorsetailHorsetail
Scientific Name: Equisetum arvense; Equisetum telmateia, Equisetum hyemale. Family: Equisetaceae.
People Use This For: Horsetail is used for diuresis, edema, kidney and bladder stones, urinary tract infections, incontinence and general disturbances of the kidney and bladder. It is also used for hair loss; tuberculosis;jaundice; hepatitis; brittle fingernails; rheumatic diseases; gout; osteoarthritis; osteoporosis; frostbite; weight loss; menorrhagia; and nasal, pulmonary, and gastric hemorrhage. Topically, horsetail is used for treatment of wounds and burns.
Safety: POSSIBLY UNSAFE ...when used orally long-term. Horsetail contains thiaminase, an enzyme that can cause thiamine deficiency. In Canada, horsetail products are required to be thiaminase-free, but there is not enough reliable information to know if thiaminase-free products are safe (3). PREGNANCY AND LACTATION: Insufficient reliable information available; avoid using.
Scientific evidence: Not enough scientific research has been done on Horsetail to comment on effectiveness.
Mechanism of Action: The applicable parts of horsetail are above ground parts. Horsetail constituents include flavonoids such as apigenin, luteolin, and kaempferol and quercetin compounds; petrosins such as onitin; caffeic acid derivatives; sterols; tannins; and saponins (6, 7, 9). Horsetail also contains significant amounts of silicon (4). Horsetail also contains trace amounts of nicotine (4). Preliminary research suggests aqueous and hydroalcoholic extracts of horsetail have antioxidant and anti-inflammatory effects (8, 10,11). Horsetail extracts might also have vasorelaxant and analgesic effects (7, 11). Other research suggests horsetail extracts might have sedative and anticonvulsant effects (8). Flavonoid and petrosin constituents might have hepatoprotective properties (6). Other preliminary research suggests that horsetail might have antiviral effects (12). Crude horsetail contains thiaminase, an enzyme that destroys thiamine (vitamin B1). Thiaminase-containing plants have been associated with neurological toxicity in animals due to thiamine deficiency (12, 14, 15). Other related horsetail (Equisetum) species have diuretic and hypoglycemic properties (16, 17, 18). Whether horsetail has these effects is unclear.
Side effects: Crude horsetail may lead to thiamine deficiency with prolonged consumption. Canadian products are required to be certified as free from thiaminase-like effect (3). Horsetail has also been associated with cross allergenicity with carrots (19). Horsetail contains tiny amounts of nicotine and may cause nicotine allergy or theoretically, nicotine toxicity if taken in large quantities (5). Topically, horsetail can cause seborrheic dermatitis (5).
Interactions with Herbs & Supplements: Betel nuts: Consuming horsetail with betel nuts might increase the risk of thiamine deficiency. Areca (betel) nuts reduce thiamine activity, probably by chemical inactivation (2). Horsetail contains thiaminase, which breaks down thiamine (13, 14, 15). CHROMIUM-CONTAINING HERBS AND SUPPLEMENTS: Horsetail contains chromium (0.0006%) and could increase the risk of chromium toxicity when taken with chromium supplements or chromium-containing herbs such as bilberry, brewer's yeast, or cascara (1). THIAMINE: Crude horsetail contains thiaminase, which breaks down thiamine. Chronic ingestion in animals has been associated with thiamine deficiency (13, 14, 15). Possible Interactions with Drugs: LITHIUM In keeping with all herbs and medications that have a diuretic action the blood levels of Lithium may be sensitive to the diuretic effect. Horsetail is thought to have diuretic properties. Theoretically, due to these potential diuretic effects, horsetail might reduce excretion and increase levels of lithium. The dose of lithium might need to be decreased.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: Diabetes: Other horsetail species have hypoglycemic activity (18). It is unclear whether horsetail has hypoglycemic effects. Until more is known, bear this in mind when using in the context of diabetes. Monitor blood sugar levels to assess any effects. Low blood potassium: Other horsetail species have diuretic activity and can increase the excretion of potassium (16, 17). Until more is known, use with caution in patients who are at risk for potassium deficiency. Thiamine deficiency: Theoretically, horsetail can cause or exacerbate thiamine deficiency (13, 14, 15). No cases have been reported in humans. For every three continuous use take a break for three weeks.
Dosage/Administration: No typical dosage.
Specific References: HORSETAIL 1. Lanca S, Alves A, Vieira AI, et al. Chromium-induced toxic hepatitis. Eur J Intern Med 2002;13:518-20. 2. Vimokesant S, Kunjara S, Rungruangsak K, et al. Beriberi caused by antithiamin factors in food and its prevention. Ann N YAcad Sci 1982;378:123-36. 3. Health Canada. Labelling Standard: Mineral Supplements. Available at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/label-etiquet-pharm/minsup_e.html (Accessed 14 November 2005). 4. Piekos R, Paslawska S. Studies on the optimum conditions of extraction of silicon species from plants with water. I. Equisetum arvense L. Herb. Planta Med 1975;27:145-50. 5. Sudan BJ. Seborrhoeic dermatitis induced by nicotine of horsetails (Equisetum arvense L.). Contact Dermatitis 1985;13:201-2. 6. Oh H, Kim DH, Cho JH, Kim YC. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J Ethnopharmacol 2004;95:421-4. 7. Sakurai N, Iizuka T, Nakayama S, et al. [Vasorelaxant activity of caffeic acid derivatives from Cichorium intybus and Equisetum arvense]. Yakugaku Zasshi 2003;123:593-8. 8. Dos Santos JG Jr, Blanco MM, Do Monte FH, et al. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia 2005;76:508-13. 9. Langhammer L, Blaszkiewitz K, Kotzorek I. Evidence of toxic adulteration of equisetum. Dtsch Apoth Ztg 1972;112:1751-94. 10. Correia H, Gonzalez-Paramas A, Amaral MT, et al. Characterisation of polyphenols by HPLC-PAD-ESI/MS and antioxidant activity in Equisetum telmateia. Phytochem Anal 2005;16:380-7. 11. Do Monte FH, dos Santos JG Jr, Russi M, et al. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol Res 2004;49:239-43. 12. Husson GP, Vilagines R, Delaveau P. [Antiviral properties of various extracts of natural origin]. Ann Pharm Fr 1986; 44:41-8. 13. Ramos JJ, Ferrer LM, Garcia L, et al. Polioencephalomalacia in adult sheep grazing pastures with prostrate pigweed. Can Vet J 2005;46:59-61. 14. Henderson JA, Evans EV, McIntosh RA. The antithiamine action of Equisetum. J Am Vet Med Assoc 1952;120:375-8. 15. Fabre B, Geay B, Beaufils P. Thiaminase activity in equisetum arvense and its extracts. Plant Med Phytother 1993;26:190-7. 16. Perez Gutierrez RM, Laguna GY, Walkowski A. Diuretic activity of Mexican equisetum. J Ethnopharmacol 1985;14:269-72. 17. Lemus I, Garcia R, Erazo S, et al. Diuretic activity of an Equisetum bogotense tea (Platero herb): evaluation in healthy volunteers. J Ethnopharmacol 1996;54:55-8. 18. Revilla MC, Andrade-Cetto A, Islas S, Wiedenfeld H. Hypoglycemic effect of Equisetum myriochaetum aerial parts on type 2 diabetic patients. J Ethnopharmacol 2002;81:117-20. 19. Agustin-Ubide MP, Martinez-Cocera C, Alonso-Llamazares A, et al. Diagnostic approach to anaphylaxis by carrot, related vegetables and horsetail (Equisetum arvense) in a homemaker. Allergy 2004;59:786-7. | |
HyssopHyssop
Hysope Officinale.
Scientific Name: Hyssopus officinalis. Family: Lamiaceae/Labiatae.
People Use This For: Hyssop is used for liver and gallbladder conditions, intestinal inflammation, coughs, the common cold, respiratory infections, sore throat, asthma, urinary tract infection, flatulence and colic, anorexia, poor circulation, painful periods, and for digestive and intestinal problems.
Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Generally Recognized as Safe (GRAS) status in the US.(12) Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: There is insufficient scientific information available about the effectiveness of hyssop.
Mechanism of Action: The applicable parts of hyssop are the above ground parts. Constituent marrubiin (13) has cardioactive effects and stimulates bronchial secretions. (14) Caffeic acid and tannins may be the active constituents of the dried leaves. Extracts show antiviral activity against herpes simplex virus and HIV in vitro. (15,13)
Adverse Reactions: None reported with tincture or infusion.
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: None known.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: None reported with tincture or infusion.
Dosage/Administration: Dr Clare’s Blends: To check Oral: Typically people take two 445 mg capsules containing the hyssop herb three times daily. (16) Some people take 10-15 drops of the hyssop extract (12-14% by volume) in water two to three times daily. (17) People also consume or gargle the hyssop tea three times daily. (18) The tea is prepared by steeping 1-2 teaspoons of the dried hyssop flower tops in 150 mL boiling water for 10-15 minutes and then straining. Avoid internal use of hyssop oil due to possible neurotoxicity.
Specific References: HYSSOP 12. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 13. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. 14. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 15. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 16. Manufacturer: Nature's Way. Springville, UT. 17. Manufacturer: Nature's Answer. Hanppange, NY. 18. Fetrow CW, Avila JR. Professional's Handbook of Complementary & Alternative Medicines. 1st ed. Springhouse, PA: Springhouse Corp., 1999. | |
J |
|---|
Jamaican DogwoodJamaican Dogwood
Also Known As: Piscidia, West Indian Dogwood.
Scientific Name: Piscidia erythrina; Piscidia communis. Family: Fabaceae/Leguminosae.
People Use This For: Jamaican dogwood is used for anxiety and fear, as a daytime sedative, for neuralgia, migraine, insomnia (especially sleeplessness due to nervous tension), and painful periods.
Safety: No concerns regarding safety. No reports of harm with therapeutic doses. Children: Refer to a Medical Herbalist. Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: There is insufficient Scientific information available about the effectiveness of Jamaican dogwood.
Mechanism of Action: The applicable part of Jamaican dogwood is the root bark. Animal studies have shown that an extract of Jamaican dogwood has sedative effects, marked cough suppressant and fever lowering activities, and also anti-inflammatory and antispasmodic action on smooth muscles. (29,30)
Adverse Reactions: None reported with therapeutic doses.
Interactions with Herbs & Supplements: Herbs and Supplements with Sedative Properties: May enhance therapeutic and adverse effects.
Interactions with Drugs: None reported. As above may enhance the effects of anxiolytic drugs.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: None known.
Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture. No typical dosage.
Specific References: JAMAICAN DOGWOOD 29. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 30. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. | |
L |
|---|
Lady's MantleLady’s Mantle. Alchemilla vulgaris
Lion's Foot, Manto de la Virgen, Nine Hooks, Nueve Ganchos, Pie de León, Silerkraut. CAUTION: See separate listing for Alpine Lady's Mantle.
Scientific Name: Alchemilla xanthochlora; Alchemilla vulgaris. Family: Rosaceae.
People Use This For: Alchemilla is used for mild diarrhoea, heavy menstrual flow, diabetes, menopausal complaints, painful menses, gastrointestinal disorders, as a relaxant for muscle spasms, an anti-inflammatory, a diuretic, and as a garglefor mouth and throat inflammation. Topically, alchemilla is used as an astringent for bleeding, to improve wound healing, for ulcers, eczema, skin rashes, and as a bath additive for treating lower-abdominal ailments.
Safety: Alchemilla has been used for many years without reports of significant toxicity (2, 3, 4). No scientific studies have been carried out for topical use of alchemilla. Pregnancy: There are no scientific studies available. A 2012 pharmaceutical review assessment describes it as safe in pregnancy Breastfeeding: There are no scientific studies available.The above review has no comment for avoiding or for indications for use.
Effectiveness: There is insufficient reliable information available about the effectiveness of alchemilla.
Mechanism of Action: The above ground parts are used. Alchemilla contains 6-8% tannins (3), which are likely to account for its perceived astringent activity (2). A water extract of Alchemilla xanthochlora demonstrates lipid peroxidation and superoxide anion scavenging activity (2). Flavonoid extracts inhibit proteolytic enzymes, including elastase, trypsin, and alpha-chymotrypsin. This property suggests alchemilla might have a role in protecting conjunctive and elastic tissues (2).
Adverse Reactions: Although one reference refers to an association with liver damage no cases have been reported and the association is likely to be spurious (3).
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: None known.
Interactions with Foods: None known.
Interactions with Lab Tests: None known
Interactions with Diseases or Conditions: None known
Dosage/Administration: Oral: For diarrhoea, a typical dose is one cup tea, prepared by steeping 1-4 grams above ground parts in boiling water for 10 minutes and then straining (3), used up to three times per day between meals. The average amount used per day is 5-10 grams. Equivalent preparations can also be used (1). Diarrhoea persisting for more than 3-4 days should be medically evaluated (3). Topical: No typical dosage.
Specific References: LADY’S MANTLE 1. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 2. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 3. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. Stuttgart: Medpharm GmbH Scientific Publishers, 1994. 4. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton,FL: CRC Press, LLC 1997 | |
Lavender
Common Lavender, English Lavender, French Lavender, Garden Lavender.
Scientific Name: Lavandula angustifolia, synonyms Lavandula officinalis, Lavandula vera, Lavandula spica; Lavandula dentata; Lavandula latifolia; Lavandula pubescens; Lavandula stoechas. Family: Lamiaceae/Labiatae.
People Use This For: Orally, lavender is used for restlessness, insomnia, nervousness, depression, meteorism (abdominal swelling from gas in the intestinal or peritoneal cavity), and loss of appetite. Lavender is also used orally for flatulence, upset stomach, giddiness, migraine headaches, toothaches, sprains, neuralgia, rheumatism, acne, sores, nausea, vomiting, to promote menstruation, and to treat cancer.
Topically, lavender is used for alopecia areata, pain, and in baths for circulation disorders, and improving psychological well-being. It is also used topically as a mosquito repellent and insect repellent.
By inhalation, lavender is used as aromatherapy for insomnia, pain, and agitation related to dementia.
In foods and beverages, lavender is used as a flavour component.
In manufacturing, lavender is utilized in pharmaceutical products and as a fragrance ingredient in soaps and cosmetics. Lavender is also used as an insect repellent.
Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Lavender has Generally Recognized as Safe (GRAS) status for food use in the US.(31). No concerns regarding safety when used orally and appropriately, (32) when used topically and appropriately. Lavender oil has been used safely for up to 7 months in adults.(33) when the essential oil is inhaled as a part of aromatherapy. (34,35,36,37)
Children: Possibly Unsafe when applied topically. Anecdotal reports suggest that applying topical products containing lavender oil to prepubertal boys can result in gynecomastia in some cases.(38) Products with a higher concentration of lavender oil and more frequent applications might be more likely to result in gynecomastia.
Pregnancy and Lactation: Insufficient reliable information available; avoid using.
Effectiveness: POSSIBLY EFFECTIVE Alopecia areata. There is some evidence that applying lavender oil in combination with the essential oils from thyme, rosemary,and cedarwood might improve hair growth by as much as 44% after 7 months of treatment.(33) INSUFFICIENT RELIABLE EVIDENCE to RATE Agitation. Evidence regarding the efficacy of lavender aromatherapy for agitation is conflicting. In one preliminary clinical study, nightly use of lavender oil in a bedside diffuser for 3 weeks reduced agitation scores in patients with various types of dementia.(36) However, continuous use of lavender oil on a pad attached to a patient's shirt had no effect in a small group of patients with advanced dementia. (35) Depression. In mild to moderate depression, tincture of lavender appears to be slightly less effective than imipramine (Tofranil). Lavender might have some additive antidepressant effect with imipramine. (32) Insomnia. Preliminary clinical research suggests using lavender oil in a vaporizer overnight might help some people with mild insomnia.(37) Psychological well-being. Preliminary clinical research suggests that adding 3 mL of a 20% lavender oil and 80% grapeseed oil mixture to daily baths produces modest improvements in mood, compared with baths containing grapeseed oil alone. (39) More evidence is needed to rate lavender for these uses.
Mechanism of Action: The applicable parts of lavender are the flowers, leaves, and oil. Lavender contains several potential active constituents including cineole from the essential oil and borneol and camphor from the leaves. (40) The oil also contains linalool, linalyl acetate, and carophyllene epoxide.(36) Lavender preparations and the isolated constituents have several pharmacological effects in vitro and in animals. However, the effects in humans are less well known. Lavender seems to induce relaxation and sedation. Lavender decreases EEG potentials and decreases alertness in humans.(41) There is some evidence that lavender has spasmolytic effects on smooth muscle (42) and might have analgesic effects.(34) There is also some evidence from animal models that lavender might have anticonvulsant effects and possibly potentiate chloral hydrate and pentobarbital effects.(41) In animal models, lavender leaf extract and essential oils seem to have analgesic and anti-inflammatory properties. (40) Lavender oil has modest estrogenic effects and antiandrogenic effects in vitro.(38) Lavender might also have stimulant effects on hair growth; (33) however, the mechanism of this effect is not known.
Adverse Reactions: Tincture of lavender may cause headache.(32)
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: May enhance the effects of sedative herbs. Advise patients th at lower than usual dose may be necessary. Refer to a Medical Adviser.
Interactions with Foods: None known.
Interactions with Lab Tests: None reported.
Interactions with Diseases or Conditions: None reported.
Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture. Oral: For depression, tincture of lavender (1:5 in 50% alcohol) 60 drops per day has been used for 4 weeks.(32) Topical: For alopecia areata, one study used a combination of essential oils including lavender 3 drops (108mg), rosemary 3 drops (114 mg), thyme 2 drops (88mg), and cedarwood 2 drops (94 mg), all mixed with 3 mL jojoba oil and 20 mL grapeseed oil. Each night, the mixture is massaged in to the scalp for 2 minutes with a warm towel placed around the head to increase absorption.(33)
For agitation associated with dementia, lavender aromatherapy has been used by applying lavender oil to a pad attached to clothingor placed in a bedside diffuser. (35,36) For insomnia, lavender aromatherapy has been used by placing lavender oil in a vaporizer. (37) For general psychological well-bei9ng, adding 3 mL of a mixture of lavender oil 20% and grapeseed oil 80% in bath water has been used.(39)
Specific References: LAVENDER 31. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 32. Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild tomoderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:123-7 33. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol 1998;134:1349-52.34. Buckle J. Use of aromatherapy as a complementary treatment for chronic pain. Altern Ther Health Med 1999;5:42-51. 35. Lynn A, Hovanec L, Brandt J. A Controlled Trial of Aromatherapy for Agitation in Nursing Home Patients with Dementia. J Alt Comp Med 2004;431-7. 36. Lin PW, Chan W, Ng BF, Lam LC. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: a cross-over randomized trial. Int J Geriatr Psychiatry 2007;22:405-10. 37. Lewith GT, Godfrey AD, Prescott P. A single-blinded, randomized pilot study evaluating the aroma of Lavandula augustifolia as a treatment for mild insomnia. J Altern Complement Med 2005;11:631-7. 38. Henley DV, Lipson N, Korach KS, Bloch CA. Prepubertal gynecomastia linked to lavender and tea tree oils. N Eng J Med 2007;356:479-85. 39. Morris N. The effects of lavender (Lavendula angustifolium) baths on psychological well-being: two exploratory randomised control trials. Complement Ther Med 2002;10:223-8. 40. Hajhashemi V, Ghannadi A, Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol 2003;89:67-71. 41. Schulz V, Hansel R, Tyler VE. Rational Phytotherapy: A Physician's Guide to Herbal Medicine. Terry C. Telger, transl. 3rd ed. Berlin, GER: Springer, 1998 42. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. | |
Lemon Balm
Melissa Scientific Name: Melissa officinalis. Family: Lamiaceae/Labiatae. People Use This For: Orally, lemon balm is used for anxiety, insomnia, dyssomnia, restlessness, dyspepsia, bloating, flatulence, colic, and for attention deficit-hyperactivity disorder (ADHD). Lemon balm is also used for Graves' disease (overactive thyroid), painful periods, cramps and headache. It is also used orally for Alzheimer's disease, melancholia, nervous palpitations, vomiting, and high blood pressure.
Topically, lemon balm is used for cold sores (herpes labialis).
Safety: No concerns regarding safety when used orally in amounts commonly found in foods.
Possibly Safe when used orally or topically and appropriately, short-term. Lemon balm has been used with apparent safety for up to 4 months.76,77,78,79,80
There is insufficient scientific information to comment about the safety of lemon balm when used long-term.
Children: Possibly Safe when used orally and appropriate, short-term. A specific combination product providing lemon balm leaf extract 80 mg and valerian root extract 160 mg (Euvegal forte, Dr. Willmar Schwabe Pharmaceuticals) 1-2 tablets once or twice daily has been safely used in children under age 12 years for about a month.81 Preliminary clinical research also suggests that a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (Colimil) is safe in infants when used for up to a week.82
Pregnancy and Lactation: Refer to a Medical Herbalist
Effectiveness: POSSIBLY EFFECTIVE Alzheimer's disease. Taking a standardized extract of lemon balm orally, daily for 4 months, seems to reduce agitation and improve symptoms of mild to moderate Alzheimer's disease on standard Alzheimer's disease rating scales.77
Colic. A clinical trial shows that breast-fed infants with colic who are given a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (Colimil) twice daily for a week have reduced crying times compared to placebo.82
Dyspepsia. A specific combination product containing lemon balm (Iberogast, Medical Futures, Inc) seems to improve symptoms of dyspepsia. The combination includes lemon balm plus peppermint leaf, German chamomile, caraway, licorice, clown's mustard plant, celandine, angelica, and milk thistle.83, 80 A meta-analysis of studies using this combination product suggests that taking 1 mL orally three times daily over a period of 4 weeks significantly reduces severity of acid reflux, epigastric pain, cramping, nausea, and vomiting compared to placebo.84
Herpes labialis (cold sores). Applying a lip balm containing 1% lemon balm extract seems to shorten healing time, prevent infection spread, and reduce symptoms of recurring cold sores.76,79
Sleep. Taking a specific combination product providing lemon balm leaf extract 80 mg and valerian root extract 160 mg (Euvegal forte, Dr. Willmar Schwabe Pharmaceuticals) three times daily appears to improve the quality and quantity of sleep in healthy people.85
INSUFFICIENT RELIABLE EVIDENCE to RATE Restless Sleep. Preliminary evidence suggests that a specific combination product providing lemon balm leaf extract 80 mg and valerian root extract 160 mg (Euvegal forte, Dr. Willmar Schwabe Pharmaceuticals) 1-2 tablets once or twice daily might decrease symptoms in children under age 12 years who have pathological restlessness.81 More evidence is needed to rate lemon balm for this use.
Mechanism of Action: The applicable part of lemon balm is the leaf. Lemon balm seems to have sedative, antioxidant, and antiviral effects.76,77,78,79 Lemon balm contains citronellal, neral, and geranial aldehydes; flavonoids and polyphenolic compounds; and monoterpene glycosides. These substances may contribute to the behavioral effects of lemon balm leaf and essential oil.78 Clinical research suggests that lemon balm induces a calming effect and reduces alertness.78
Adverse Reactions: Orally, lemon balm is well tolerated. Rarely it may cause nausea, vomiting, abdominal pain, dizziness, and wheezing.77
Topically, there is one report of irritation and one report of exacerbation of herpes symptoms when lemon balm was applied.76
Interactions with Herbs & Supplements: Additive effect with other nervine (relaxing) herbs.
Interactions with Drugs: CNS Depressants: Theoretically, concomitant use of lemon balm with drugs with sedative properties may cause additive effects and side effects.78
Interactions with Foods: None reported
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: Thyroid Disorders: In laboratory studies thyroid hormone release is affected by Lemonbalm. No reported clinical cases.
Glaucoma: Animal studies incicate there may be a problem. No reported clinical cases.
Surgery: Tell patients to discontinue lemon balm at least 2 weeks before elective surgical procedures.
Dosage/Administration: Dr Clare’s Blends: 1 gm per day
Oral: For mild to moderate Alzheimer's disease, 60 drops per day of a standardized lemon balm extract, prepared 1:1 in 45% alcohol, has been used.77
For improving sleep in healthy adults, a specific combination product providing lemon balm leaf extract 80 mg and valerian root extract 160 mg (Euvegal forte, Dr. Willmar Schwabe Pharmaceuticals) 3 times daily has been used for up to 30 days.85
For colic in infants, a specific multi-ingredient product containing fennel 164 mg, lemon balm 97 mg, and German chamomile 178 mg (Colimil) twice daily for a week has been used,82
For dyspepsia, a specific combination product containing lemon balm (Iberogast, Medical Futures, Inc) and several other herbs has been used in a dose of 1 mL three times daily.83,80,84 For dyssomnia in children, a specific combination product providing lemon balm leaf extract 80 mg and valerian root extract 160 mg (Euvegal forte, Dr. Willmar Schwabe Pharmaceuticals) 1-2 tablets once or twice daily has been used.81
Topical: For herpes labialis (cold sores), the cream or ointment containing 1% of a 70:1 lyophilized aqueous extract is usually applied two to four times daily from first symptom to a few days after the lesions have healed.76,79
Specific References: LEMON BALM 76. Wolbling RH, Leonhardt K. Local therapy of herpes simplex with dried extract from Melissa officinalis. Phytomedicine 1994;1:25-31. 77. Akhondzadeh S, Noroozian M, Mohammadi M, et al. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomised, placebo controlled trial. J Neurol Neurosurg Psychiatry 2003;74:863-6. 78. Kennedy DO, Scholey AB, Tildesley NT, et al. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol Biochem Behav 2002;72:953-64. 79. Koytchev R, Alken RG, Dundarov S. Balm mint extract (Lo-701) for topical treatment of recurring herpes labialis. Phytomedicine 1999;6:225-30. 80. Madisch A, Holtmann G, Mayr G, et al. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004;69:45-52. 81. Muller SF, Klement S. A combination of valerian and lemon balm is effective in the treatment of restlessness and dyssomnia in children. Phytomedicine 2006;13:383-7. 82. Savino F, Cresi F, Castagno E, et al. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytother Res 2005;19:335-40. 83. Holtmann G, Madisch A, Juergen H, et al. A double-blind, randomized, placebo-controlled trial on the effects of an herbal preparation in patients with functional dyspepsia [Abstract]. Ann Mtg Digestive Disease Week 1999 May. 84. Melzer J, Rosch W, Reichling J, et al. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther 2004;20:1279-87. 85. Cerny A, Shmid K. Tolerability and efficacy of valerian/lemon balm in healthy volunteers (a double blind, placebo-controlled, multicentre study). Fitoterapia 1999;70:221-8. | |
Lime Flower (LINDEN)
European Linden, Lime Flower, Lime Tree, Linden Charcoal, Tila, Scientific Name: Tilia europaea, Tilia cordata. Family: Malvaceae or Tiliaceae.
People Use This For: Linden flower and flower bract is used for colds, nasal congestion, throat irritation, palpitations, hypertension, headaches, insomnia, sinus headache, migraine headache, incontinence, hemorrhage, arteriosclerotic hypertension, fever, and nervous tension. It is also used to induce sweating, diuretic, antispasmodic, and as an expectorant for coughs.
Safety: No concerns regarding safety when used orally and appropriately.43
Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: There is insufficient scientific information available to comment.
Mechanism of Action: The applicable parts of linden are the dried flower and flower bract.
Linden has antispasmodic, diaphoretic, diuretic, sedative, mild astringent, and antifungal activity.44,45
In vitro, its antispasmodic activity is attributed to p-coumaric acid and the flavonoid constituents.44 Diaphoretic effects are thought to be due to kaempferol, p-coumaric acid, and quercetin constituents.45
The volatile oils, including citral, citronellal, citronellol, eugenol, and limonene, exert sedative and antispasmodic effects.44,45
Adverse Reactions: None known.
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: Lithium
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture.
Oral: Traditionally 1-2 cups of the tea has been used. Additionally, 2-4 mL of the tincture (1:5 in 45% alcohol), and 1-2 mL of the liquid extract (1:1 in 25% alcohol) is 1-2 mL has also been used.44
Specific References: LINDEN 43. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 44. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 45. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. | |
Liquorice
Chinese Licorice. Scientific Name: Glycyrrhiza glabra; Family: Fabaceae/Leguminosae. People Use This For: Licorice is used for stomach and duodenal ulcers, sore throat, bronchitis, gastritis, indigestion, colic, insufficiency of the adrenal cortex and cough. In combination with Panax ginseng and Bupleurum falcatum, licorice is used orally to help stimulate adrenal gland function, particularly in patients with a history of long-term corticosteroid use. As a component of the herbal formula, Shakuyaku-Kanzo-To, licorice is used to increase fertility in women with polycystic ovary syndrome. In combination with other herbs, licorice is used to treat prostate cancer and eczema.
Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Possibly Safe when used orally and appropriately for medicinal purposes in the short-term.23,24,25,26,29,32 Long-term use increases the risk of side effects such as hypertension and low potassium33 in susceptible people. Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: POSSIBLY EFFECTIVE Dyspepsia. A specific combination product containing licorice (Iberogast, Medical Futures, Inc) seems to improve symptoms of dyspepsia. The combination includes licorice plus peppermint leaf, German chamomile, caraway, lemon balm, clown's mustard plant, celandine, angelica, and milk thistle.30,27 A meta-analysis of studies using this combination product suggests that taking 140 mL orally three times daily over a period of 4-weeks significantly reduces severity of acid reflux, stomach pain, cramping, nausea, and vomiting compared to placebo.31
INSUFFICIENT SCIENTIFIC EVIDENCE to VERIFY: Muscle cramps. Preliminary clinical research suggests taking a specific combination of licorice and peony may reduce muscle cramps in patients with hepatic cirrhosis or in patients undergoing hemodialysis.34,35,36
Peptic ulcers. There is some evidence that deglycyrrhizinated licorice might accelerate the healing of peptic ulcers.29,32
Weight loss. There is conflicting information about the use of licorice for weight loss. Licorice has been shown to reduce body fat, however accompanying fluid retention offsets any change in body weight.26 More evidence is needed to rate licorice for these uses. Mechanism of Action: The applicable part of licorice is the root. Licorice has antispasmodic, anti-inflammatory, laxative, and soothing properties.
Licorice appears to block metabolism of prostaglandins linked to inflammation, which suggests the possible beneficial effect on peptic ulcer. Cortisol promotes sodium and water retention and potassium excretion.28,37,38,41Excessive licorice ingestion can therefore produce a syndrome of apparent excess of adrenal cortical hormones leading to increased urinary potassium loss and hypertension.42,43,44,45,46,39,40,41 There is considerable variation in the amount of licorice needed to cause these effects, due in part to variation in the glycyrrhizic acid content of licorice preparations. There is also variation in people's response to licorice. Those with hypertension, heart disease, kidney disease, or a high salt intake are more sensitive to its effects.47,37,40,41 Finally, case reports of adverse reactions to licorice do not always make it clear whether licorice intake is in grams of pure licorice, or grams of sweet licorice candy or salty licorice or another preparation. The increases in blood pressure, and cortisol to cortisone ratio are proportional to the amount of glycyrrhizic acid ingested.33 Licorice appears to have anti-estrogenic and estrogenic action. Preliminary research indicates that licorice does not stimulate the growth of estrogen dependent breast cancer cells.48 However, the estrogenic effects of licorice might be concentration dependent. Glabridin, an isoflavone constituent of licorice, seems to have an estrogen receptor-dependent growth-promoting effect at low concentrations. At higher concentrations, it seems to have an estrogen receptor-independent antiproliferative effect.51 Adverse Reactions: Orally. excessive licorice ingestion can cause a problem with overproduction of adrenal cortex hormones.42,28,43,44,37,38,45,46,39,40,41,52,53 These effects are most likely to occur when 30 grams or more of licorice is consumed daily for several weeks.42,49,4438,46,39,50,41,53 Interactions with Herbs & Supplements: Cardiac Glycoside-Containing Herbs: None commonly found in products in Ireland. Stimulant Lasative Herbs: Excessive amounts can be an issue. Interactions with Drugs: Antihypertensive Drugs: Refer to Medical Herbalist Corticosteroids: Refer to Medical Herbalist Digoxin: Refer to Medical Herbalist Diuretic Drugs: Refer to Medical Herbalist. Estrogens: Avoid high dosages and prolonged treatment.R1 pp.474 Warfarin: (Coumadin)
Interactions with Foods: Grapefruit Juice: Theoretically, grapefruit juice and its component naringenin might enhance the mineralocorticoid activities of licorice, by blocking the conversion of cortisol to cortisone.54,55
Salt: A high salt diet can exacerbate adverse effects of licorice such as sodium and water retention and hypertension.41 Interactions with Lab Tests: 1407-Hydroxyprogesterone: Licorice can increase serum 1407-hydroxyprogesterone concentrations and test results in healthy volunteers who consume 7 grams of licorice per day.56,57
Potassium: Excessive use of licorice can affect Potassium levels Interactions with Diseases or Conditions: Heart Disease: Refer to a Medical Herbalist. Hormone Sensitive Cancers/Conditions: As above. Hypertension: Advise patients with hypertension to avoid excessive amounts of licorice.58,59 Kidney Insufficiency: Advise patients with severe renal insufficiency to avoid excessive amounts of licorice.60 Surgery: discontinue licorice 2 weeks before elective surgical procedures. Dosage/Administration: Dr Clare’s Blends: has a recommended dose of 5mls per week i.e 140.4mls per day of 140:3 extract = ½ gram daily. Oral: For dyspepsia, a specific combination product containing licorice (Iberogast, Medical Futures, Inc) and several other herbs has been used in a dose of 140 mL three times daily.30,27,31 2-6ml/day of 140:140 extract 6-1402ml/day of 140:3 extract Dried Root 140-4gms per day. Dr Clare’s Comment: The effect of liquorice on the Adrenal Glands is beneficial for most patients, however a small group of patients are sensitive to the effects on blood pressure. Under normal circumstances this would not be significant for short term low dose use. It can be monitored by taking blood pressure and many chemists have a blood pressure machine patients can check during treatment if you do not have resources to check the blood pressure. Specific References: LICORICE 23. Abe Y, Ueda T, Kato T, Kohli Y. [Effectiveness of interferon, glycyrrhizin combination therapy in patients with chronic hepatitis C]. [Article in Japanese]. Nippon Rinsho 140994;52:14081407-22. 24. Acharya SK, Dasarathy S, Tandon A, et al. A preliminary open trial on interferon stimulator (SNMC) derived from Glycyrrhiza glabra in the treatment of subacute hepatic failure. Indian J Med Res 140993;98:69-74. 25. Zhang XH, Lowe D, Giles P, et al. Gender may affect the action of garlic oil on plasma cholesterol and glucose levels of normal subjects. J Nutr 200140;1403140:14047140-8. 26. Armanini D, De Palo CB, Mattarello MJ, et al. Effect of licorice on reduction of body fat mass in healthy subjects. J Endocrinol Invest 2003;26:646-50. 27. Madisch A, Holtmann G, Mayr G, et al. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004;69:45-52. 28. Hussain RM. The sweet cake that reaches parts other cakes can't! Postgrad Med J 2003;79:1401405-6. 29. Turpie AG, Runcie J, Thomson TJ. Clinical trial of deglydyrrhizinized liquorice in gastric ulcer. Gut 140969;1400:299-302. 30. Holtmann G, Madisch A, Juergen H, et al. A double-blind, randomized, placebo-controlled trial on the effects of an herbal preparation in patients with functional dyspepsia [Abstract]. Ann Mtg Digestive Disease Week 140999 May. 31. Melzer J, Rosch W, Reichling J, et al. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther 2004;20:140279-87. 32. Tewari SN, Wilson AK. Deglycyrrhizinated liquorice in duodenal ulcer. Practitioner 140973;21400:820-3. 33. Sigurjonsdottir HA, Franzson L, Manhem K, et al. Liquorice-induced rise in blood pressure: a linear dose-response relationship. J Hum Hypertens 200140;1405:549-52. 34. Kumada T, et al. Effect of Shakuyaku-kanzo-to (Tsumura TJ-68) on muscle cramps accompanying cirrhosis in a placebo-controlled double-blind parallel study. J Clin Ther Med 140999;1405:499-523. 35. Hyodo T, Taira T, Kumakura M, et al. The immediate effect of Shakuyaku-kanzo-to, traditional Japanese herbal medicine, for muscular cramps during maintenance hemodialysis. Nephron 2002;90:240 36. Hinoshita F, Ogura Y, Suzuki Y, et al. Effect of orally administered shao-yao-gan-cao-tang (Shakuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: a preliminary study. Am J Chin Med 2003;3140:445-53. 37. Elinav E, Chajek-Shaul T. Licorice consumption causing severe hypokalemic paralysis. Mayo Clin Proc 2003;78:767-8. 38. Eriksson JW, Carlberg B, Hillom V. Life-threatening ventricular tachycardia due to liquorice-induced hypokalemia. J Intern Med 140999;245:307-1400. 39. van den Bosch AE, van der Klooster JM, Zuidgeest DM, et al. Severe hypokalemic paralysis and rhabdomyolysis due to ingestion of liquorice. Neth J Med 2005;63:14046-8. 40. van Uum SH. Liquorice and hypertension. Neth J Med 2005;63:1401409-20. 41. Stormer FC, Reistad R, Alexander J. Glycyrrhizic acid in liquorice - evaluation of health hazard. Food Chem Toxicol 140993;3140:303-1402. 42. Farese RV Jr, Biglieri EG, Shackleton CH, et al. Licorice-induced hypermineralocorticoidism. N Engl J Med 14099140;325:140223-7. 43. de Klerk GJ, Nieuwenhuis G, Beutler JJ. Hypokalemia and hypertension associated with use of liquorice flavoured chewing gum. BMJ 140997;31404:73140-2. 44. Dellow EL, Unwin RJ, Honour JW. Pontefract cakes can be bad for you: refractory hypertension and liquorice excess. Nephol Dial Transplant 140999;1404:21408-20. 45. Janse A, van Iersel M, Hoefnagels WH, Olde Rikker MG. The old lady who liked liquorice: hypertension due to chronic intoxication in a memory-impaired patient. Neth J Med 2005;63:14049-50. 46. Lin SH, Yang SS, Chau T, Halperin ML. An unusual cause of hypokalemic paralysis: chronic licorice ingestion. Am J Med Sci 2003;325:14053-6. 47. Yasue H, Itoh T, Mizuno Y, Harada E. Severe hypokalemia, rhabdomyolysis, muscle paralysis, and respiratory impairment in a hypertensive patient taking herbal medicines containing licorice. Intern Med 2007;46:575-8. 48. Amato P, Christophe S, Mellon PL. Estrogenic activity of herbs commonly used as remedies for menopausal symptoms. Menopause 2002;9:14045-50. 49. Brayley J, Jones J. Life-threatening hypokalemia associated with excessive licorice ingestion (letter). Am J Psychiatry 140994;1405140:61407-8. 50. Russo S, Mastropasqua M, Mosetti MA, et al. Low doses of liquorice can induce hypertension encephalopathy. Am J Nephrol 2000;20:14045-8. 51. Tamir S, Eizenberg M, Somjen D, et al. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res 2000;60:5704-9. 52. Sontia B, Mooney J, Gaudet L, Touyz RM. Pseudohyperaldosteronism, liquorice, and hypertension. J Clin Hypertens (Greenwich) 2008;1400:14053-7. 53. Lapi F, Gallo E, Bernasconi S, et al. Myopathies associated with red yeast rice and liquorice: spontaneous reports from the Italian Surveillance System of Natural Health Products. Br J Clin Pharmacol 2008;66:572-4. 54. Lee YS, Lorenzo BJ, Koufis T, et al. Grapefruit juice and its flavonoids inhibit 140140 beta-hydroxysteroid dehydrogenase. Clin Pharmacol Ther 140996;59:62-7140. 55. Zhang YD, Lorenzo B, Reidenberg MM. Inhibition of 140140 beta hydroxysteroid dehydrogenase obtained from guinea pig kidney by furosemide, naringenin and some other compounds. J Steroid Biochem Mol Biol 140994;49:8140-5. 56. Armanini D, Bonanni G, Palermo M, et al. Reduction of serum testosterone in men by licorice. N Engl J Med 140999;34140:14014058. 57. Armanini D, Bonanni G, Mattarello MJ, et al. Licorice consumption and serum testosterone in healthy man. Exp Clin Endocrinol Diabetes 2003;140140140:34140-3. 58. Sigurjonsdottir HA, Ragnarsson J, Franzson L, Sigurdsson G. Is blood pressure commonly raised by moderate consumption of liquorice? J Hum Hypertens 140995;9:345-8. 59. Francini-Pesenti F, Puato M, Piccoli A, Brocadello F. Liquorice-induced hypokalaemia and water retention in the absence of hypertension. Phytother Res 2008;22:563-5. 60. Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. J Clin Endocrinol Metab 2003;88:2384-92. | |
M |
|---|
Marshmallow leaf & root
Althaeae Folium, Althaeae Radi, Herba Malvae. Scientific Name: Althaea officinalis. Family: Malvaceae. People Use This For: Orally, marshmallow leaf and root are used for respiratory tract mucous membrane inflammation, dry cough, inflammation of the gastric mucosa, diarrhea, peptic ulcers, constipation, urinary tract inflammation. Safety: No concerns regarding safety when used in amounts commonly found in foods. Marshmallow root has Generally Recognized As Safe status (GRAS) for use in foods in the US.123 No concerns regarding safety when used orally in medicinal amounts.124,125 No concerns regarding safety when used topically.124 Pregnancy and Lactation: Insufficient reliable information available. Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available about the effectiveness of marshmallow. Mechanism of Action: The applicable parts of marshmallow are the leaves and the root. Marshmallow leaf and root contain mucilage sugars that can soothe and protect mucous membranes from local irritation by forming a protective layer.126,124,127,128,129,130 The mucilage can inhibit mucociliary transport,126,127,130 stimulate ingestion of breakdown products by the cell,126,130 suppress cough,126,127,131,132 increase the anti-inflammatory effects of topical steroids126,127,130 and have blood sugar lowering effect.126,124 The mucilage can also have antimicrobial, anti-spasm, antisecretory, diuretic, and wound-healing effects.124,127 Adverse Reactions: None reported. Interactions with Herbs & Supplements: None known. Interactions with Drugs: The mucilage in marshmallow might impair absorption of oral drugs.126,132,125,133 Interactions with Foods: None known. Dosage/Administration: Dr Clare’s Blends: 1gm/day. Oral: For irritation of the mouth or pharynx and associated dry cough, the typical dose of marshmallow is 2-5 grams of the dried leaf, 5 grams of the dried root, or one cup of either leaf or root tea three times daily.124 Sprecific References: MARSHMALLOW 123. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 124. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 125. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 126. Monographs on the medicinal uses of plant drugs. Exeter, UK: European Scientific Co-op Phytother, 1997. 127. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 128. Schulz V, Hansel R, Tyler VE. Rational Phytotherapy: A Physician's Guide to Herbal Medicine. Terry C. Telger, transl. 3rd ed. Berlin, GER: Springer, 1998. 129. Martindale W. Martindale the Extra Pharmacopoeia. Pharmaceutical Press, 1999. 130. Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. 1st ed. Montvale, NJ: Medical Economics Company, Inc., 1998. 131. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. Stuttgart: Medpharm GmbH Scientific Publishers, 1994. 132. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. 133. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. | |
Meadowsweet Filipendula
Scientific Name: Filipendula ulmaria, Filipendula spiraea. Family: Rosaceae. Parts used: Flowers, stems, leaves. Traditional use. Meadowsweet is used for colds, bronchitis, dyspepsia, heartburn, peptic ulcer disease, and rheumatic disorders including gout. It is also used as a diuretic and urinary antiseptic for acute cystitis.
Safety. There are no safety concerns when used appropriately. (4) One case report on the use of a blend of herbs including meadowsweet has been reported in a child presenting with bleeding from the upper digestive system.(15) As tannins precipitate proteins it is suggested that it is taken between meals if you have a low protein diet. This also applies to tea, red wine and dark chocolate. I have not witnessed any such concerns in clinical practice. Theoretically salicylates may be associated with Reyes syndrome although no cases have been reported with meadowsweet.
Breastfeeding; Consult a medical herbalist.
Constituents. Volatile oils containing salicylaldehyde, ethylsalicylate, methylsalicylate, methoxybenzaldehyde and others. Phenolic glycosides; spirein, monotropitin (gaultherin), these are the primeverosides of salicyl aldehyde and methyl methyl salicylate.; also isosalicin. Flavonoids; spiraeoside, rutin, quercitin, hyperoside, avicularin. Tannins (polyphenols); mainly hydrolysable tannins. Miscellaneous; phenylcarboxylic acids, traces of coumarin, ascorbic acid (vitamin C). Scientific evidence. No clinical studies have been done. Mechanism of action. Meadowsweet has stomachic, mild urinary antiseptic, diuretic, anti-rheumatic, astringent, and antacid activities.(1) In laboratory studies meadowsweet demonstrates anti-inflammatory effects on pro-inflammatory mediators (cytokines) and on scavenger cells.([1],7,8) Meadowsweet is a rich source of anti-oxidants.(14)It contains tannins and salicin, a plant salicylate. (2,4) In animals, meadowsweet decreases motor activity, lowers temperature, induces muscle relaxation, and increases the effect of codeine related pain relieving substances. (1) In animals the flower extract increases life expectancy, decreases vascular permeability, increases bronchial, intestinal, and uterine tone and promotes uric acid excretion. In laboratory studies it inhibits the growth of bacteria (bacteriostatic activity).(1) Water extracts of Meadowsweet contain high concentrations of tannins with astringent effects. (1)Meadowsweet exhibited protective properties in liver cells exposed to toxins.(6) This extract produced a normalizing effect on activity of protective enzymes including markers of cell breakdown, lipid peroxidation, and antioxidant defense system in liver cells. In animal studies meadowsweet demonstrated a positive effect on renal blood flow resulting in a diuretic effect.(9) Interestingly a recent study showed an anxiolytic effect of Meadowsweet extract on mice, this is in keeping with the herbal tradition of digestive health being central to your sense of well-being.(10) In laboratory research meadowsweet demonstrated anti-bacterial actions including H. Pylori which is implicated in the pathology of peptic ulcers.(11,13) Meadowsweet demonstrates inhibitory properties on the enzyme xanthine oxidase which is the primary target for the treatment of gout. (12)
Adverse Reactions. For a small percentage of people Meadowsweet can cause minor digestive problems.(5) There is no way to predict this except they are often people who have poor tolerance to prescription drugs. Wheeze (bronchospasm) has rarely been reported.(1)
In keeping with Willow Bark there may theoretically be side effects of overall increased salicylic acid exposure. This is unlikely with low dose aspirin but vigilance is recommended.
Interactions with herbs and supplements: None known.
Interactions with Drugs. Aspirin. Salicylate drugs as described. Salicin doesn't seem to have the antiplatelet effects of aspirin on blood clotting. (4) Salicylate medicationMeadowsweet contains salicin, a plant salicylate. Theoretically, meadowsweet might have an additive effect with other salicylate-containing drugs such as aspirin, NSAIDs andcholine magnesium trisalicylate. (4) Narcotic drugs e.g. codeine. Theoretically, meadowsweet can enhance the effects of codeine like drugs. (1)
Interactions with foods. None known.
Interactions with laboratory tests. None known.
Interactions with diseases or conditions. Aspirin allergy: Use meadowsweet cautiously in individuals with aspirin allergy because of salicylate constituents. Asthma: In theory meadowsweet might exacerbate asthma due to bronchospastic effects. Observe for effects on symptoms if you are asthmatic. (1) In fifteen years of herbal medicine practice I have not observed this effect nor has it been reported by others, but it is worth bearing in mind. Dosage. Recommended dose: 6-12mls per day 1:5 tincture 30% alcohol. Infusion: range from 1-1½ tsps. per day. Powder/capsule: range from 1.5-3gms per day. Liquid extract: 2-6mls 1:2 in 30% alcohol per day. Dr. Clare’s Blend: ½ tsp. per day.
References:
Phenolic Extracts from Meadowsweet and Hawthorn Flowers Have Antioxidative Properties. Zbigniew Sroka, Wojciech Cisowski, Magdalena Seredyn ́ska and Maria Łuczkiewicz. Z. Naturforsch, 56c, 739Ð744 (2001); received July 13, 2000/April 6, 2001. 6. I. V. Shilova, T. V. Zhavoronok, N. I. Souslov, T. P. Novozheeva, R. N. Mustafin, A. M. Losseva. Hepatoprotective properties of fractions from meadowsweet extract during experimental toxic hepatitis. Bulletin of Experimental Biology and Medicine. July 2008, Volume 146, Issue 1, pp 49-51. 7. Elaine M Drummond, Niamh Harbourne, Eunice Marete, Danika Martyn, JC Jacquier, Dolores O'Riordan andEileen R Gibney.. Inhibition of Proinflammatory Biomarkers in THP1 Macrophages by Polyphenols Derived From Chamomile, Meadowsweet and Willow bark. Phytotherapy Research Volume 27, Issue 4, pages 588–594, April 2013 8. Elaine M. Drummond, Harbourne N, Marete E, Jacquier J.C, O'Riordan D, Gibney E.R. An In Vivo Study Examining the Antiinflammatory Effects of Chamomile, Meadowsweet, and Willow Bark in a Novel Functional Beverage. Journal of dietary Supplements. December 2013, Vol. 10, No. 4 , Pages 370-380.
9. Bernatoniene J, Savicka A, Bernatoniene J, Kalvéniené Z, Klimas R. Kaunas University of Medicine. The Effect of Meadowsweet (Filipendula ulmaria) Flower Extract and Hydrothiazide on Renal Physiological Function in Rats. Book: Functional Foods for Chronic Diseases.
10. V. V. Udut, A. I. Vengerovskii, N. I. Suslov, I. V. Shilova, A. V. Kaigorodtsev, N. Yu. Polomeeva, A. M. Dygai. Pharmaceutical Chemistry Journal. November 2012, Volume 46, Issue 8, pp 492-494 Anxiolytic activity of biologically active compounds from Filipendula vulgaris 11. RauhaJP, RemesS, HeinonenM et al. Anu Hopiab, Marja Kähkönenb, Tytti Kujalac, Kalevi Pihlajac, Heikki Vuorelaa, Pia Vuorela. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology. Volume 56, Issue 1, 25 May 2000, Pages 3–12
12. Kazazi F, Halkes SBA, Quarles van Ufford HV, Beukelman CJJ, Van den Berg AJJ. Inhibition of xanthine oxidase activity by Filipendula species. Planta Med 2009; 75 - PA3 13. Cwikla C, K Schmidt K, Matthias A, KM Bone KM, RP Lehmann RP, E Tiralongo E. Investigations into the antibacterial activities of herbal medicines against Helicobacter pylori and Campylobacter jejuni. Planta Med 2008; 74 - PA103. 14. Barros L, Cabrita L, Vilas Boas M, Carvalho AM, Ferreira ICFR. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chemistry. Volume 127, Issue 4, 15 August 2011, Pages 1600–1608. 15. Annali dell'Istituto Superiore di Sanità Ann. Ist. Super. Sanità vol.47 n.3 Roma Jan. 2011. [1] Phytother Res. 2013 Apr;27(4):588-94. Inhibition of proinflammatory biomarkers in THP1 macrophages by polyphenols derived from chamomile, meadowsweet and willow bark. Drummond EM, Harbourne N, Marete E et al. | |
Milk Thistle
Blessed Milk Thistle, Carduus Marianum, Holy Thistle, Silybum, Silymarin, St. Mary Thistle, St. Marys Thistle.
Scientific Name: Silybum marianum, synonym Carduus marianus. Family: Asteraceae/Compositae.
People Use This For: Milk thistle is used for liver disorders including toxic liver damage caused by chemicals, Amanita phalloides mushroom poisoning, jaundice, chronic inflammatory liver disease, hepatic cirrhosis, and chronic hepatitis. It is also used orally for loss of appetite, dyspepsia and gallbladder complaints, diabetes, hangover, and diseases of the spleen. Milk thistle is used orally for stimulating breast milk flow, and stimulating menstrual flow.
In foods, the milk thistle leaves and flowers are eaten as a vegetable and seeds are roasted for use as a coffee substitute.
Safety: No concerns regarding safety when used orally and appropriately. Milk thistle extracts standardized to contain 70% to 80% of the silymarin constituent seems to be safe when used for up to 41 months.38,39,40,41,42,43,44,45
Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: POSSIBLY EFFECTIVE Diabetes. Taking the milk thistle constituent silymarin 200 mg three times daily for 4 months, in combination with conventional treatment, appears to significantly decrease fasting blood glucose, hemoglobin A1c (HbA1c), total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides compared to placebo in patients with type 2 diabetes.45 Other preliminary evidence suggests that silymarin 200 mg three times daily reduces insulin resistance in people with coexisting diabetes and alcoholic cirrhosis.46
Dyspepsia. A specific combination product containing milk thistle (Iberogast, Medical Futures, Inc) seems to improve symptoms of dyspepsia. The combination includes milk thistle plus peppermint leaf, German chamomile, caraway, licorice, clown's mustard plant, celandine, angelica, and lemon balm.47,48 A meta-analysis of studies using this combination product suggests that taking 1 mL orally three times daily over a period of 4 weeks significantly reduces severity of acid reflux, epigastric pain, cramping, nausea, and vomiting compared to placebo.49
INSUFFICIENT SCIENTIFIC EVIDENCE to COMMENT Alcohol-related liver disease.
Hepatitis B or Hepatitis C.
More evidence is needed to rate milk thistle for these uses.
Mechanism of Action: The applicable parts of milk thistle are the seed and above ground parts. The seed is most commonly used medicinally. Silymarin, the active constituent of the milk thistle seed, consists of four flavonolignans called silibinin (silybinin, silybin), isosilybinin, silichristin (silychristin), and silidianin. Silibinin makes up about 70% of silymarin.50,51 When ingested, silymarin undergoes enterohepatic recirculation and has higher concentrations in liver cells.
Adverse Reactions: Milk thistle is usually well-tolerated.52,51
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: Tamoxifen (Nolvadex): (Used for breast cancer). Theoretical risk. Refer to a Medical Herbalist.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Dosage/Administration: For diabetes, silymarin 200 mg three times daily has been used in combination with conventional treatment.46,45
References: MILK THISTLE 38. Szilard S, Szentgyorgyi D, Demeter I. Protective effect of Legalon in workers exposed to organic solvents. Acta Med Hung 1988;45:249-56. 39. Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol 1989;9:105-13. 40. Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind, controlled study. Scand J Gastroenterol 1982;17:517-21. 41. Bunout D, Hirsch S, Petermann M. [Controlled study of the effect of silymarin on alcoholic liver disease.] [Article in Spanish]. Rev Med Chil 1992;120:1370-5. 42. Trinchet JC, Coste T, Levy VG. [Treatment of alcoholic hepatitis with silymarin. A double-blind comparative study in 116 patients]. [Article in French]. Gastroenterol Clin Biol 1989;13:120-4. 43. Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol 1998;28:615-21. 44. Rambaldi A, Jacobs B, Iaquinto G, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C virus liver diseases. Cochrane Database Syst Rev 2005;2:CD003620. 45. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res 2006;20;1036-9. 46. Velussi M, Cernigoi AM, De Monte A, et al. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol 1997;26:871-9. 47. Holtmann G, Madisch A, Juergen H, et al. A double-blind, randomized, placebo-controlled trial on the effects of an herbal preparation in patients with functional dyspepsia [Abstract]. Ann Mtg Digestive Disease Week 1999 May. 48. Madisch A, Holtmann G, Mayr G, et al. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004;69:45-52. 49. Melzer J, Rosch W, Reichling J, et al. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther 2004;20:1279-87. 50. Venkataramanan R, Ramachandran V, Komoroski BJ, et al. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab Dispos 2000;28:1270-3. 51. Boerth J, Strong KM. The clinical utility of milk thistle (Silybum marianum) in cirrhosis of the liver. J Herb Pharmacother 2002;2:11-7. 52. Anon. Milk thistle: Effects on liver disease and cirrhosis and clinical adverse effects. Summary, Evidence Report/Technology Assessment: Number 21, September 2000. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/clinic/epcsums/milktsum.htm 53. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res 2006;20;1036-9. | |
Motherwort
Leonuri cardiacae herba, Mother's Wort. Scientific Name: Leonurus cardiaca. Family: Lamiaceae/Labiatae. People Use This For: Motherwort is used for heart symptoms of anxiety, cardiac insufficiency, fast heart rate or other irregular heart beats, absence of periods, flatulence, and overactive thyroid. Safety: No connerns regarding safety when used orally and appropriately.80 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available to comment on the effectiveness of motherwort. Mechanism of Action: The applicable parts of motherwort are the above ground parts. Motherwort has sedative,81,82 reduces the over-excitability of the heart. Adverse Reactions: None reported. Interactions with Herbs & Supplements: None known. Interactions with Foods: None known. Interactions with Lab Tests: THYROID FUNCTION: Motherwort might improve thyroid function and thyroid function test results in patients with thyroid overactivity.83 Refer to Medical Herbalist. Dosage/Administration: Dr Clare’s Blends: 1 gm per day. Oral: 2-4gms/day (British Herbak Pharmacopeia)1983). Specific References: MOTHERWORT 81. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 80. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 82. Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. 1st ed. Montvale, NJ: Medical Economics Company, Inc., 1998. 83. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. | |
N |
|---|
Nettle Leaf
Common Nettle, Nettle Leaf, Urtica. Scientific Name: Urtica dioica; Family: Urticaceae.
People Use This For: Stinging nettle above ground parts is used for allergies, allergic rhinitis, and musculoskeletal disease such as osteoarthritis. It is also used orally in conjunction with copious fluid intake in so-called "irrigation therapy" for urinary tract infections, urinary tract inflammation, and kidney stones. People also use the above ground parts of stinging nettle for internal bleeding, including uterine bleeding, epistaxis, and melena; anemia; poor circulation; splenomegaly; diabetes and other endocrine disorders; gastric hyperacidity; biliary complaints; diarrhea and dysentery; asthma; pulmonary congestion; rash and eczema; cancer; prevention of signs of aging; blood purification; wound healing; and as a general tonic.
In foods, young stinging nettle leaves are eaten as a cooked vegetable.
In manufacturing, stinging nettle extract is used as an ingredient in hair and skin products.
Safety: No concerns regarding safety when used orally and appropriately.
Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Allergic rhinitis (hayfever). There is preliminary evidence that stinging nettle above ground parts might improve symptoms of allergic rhinitis. Starting stinging nettle at the first sign of symptoms seems to provide subjective improvement.22
Osteoarthritis. There is evidence that oral or topical use of stinging nettle leaf extract might improve symptoms of pain in patients with osteoarthritis.23,24 Some clinicians use stinging nettle leaf extract in combination with conventional nonsteroidal anti-inflammatory drugs (NSAIDs) or other analgesics. Evidence suggests that adding stinging nettle might allow for using lower analgesic doses in some patients.24 Topically, stinging nettle leaf seems to improve pain and disability in patients with osteoarthritis of the thumb, according to preliminary research.25
More evidence would be helpful to rate stinging nettle for these uses.
Mechanism of Action: The applicable parts of stinging nettle are the above ground parts. Stinging nettle leaves contain several nutrients and active constituents. The leaves are eaten as a food because of significant amounts carotene, vitamin C, vitamin K, potassium, and calcium.26,27,28,29 There is about as much vitamin C and carotene in stinging nettle leaves as in spinach and other greens.30 The leaves also contain beta-sitosterol and the flavonoids quercetin, rutin, kaempferol, and others. Stinging nettle tops seems to have a variety of pharmacological effects including analgesic,23,31 anti-inflammatory,29 local anesthetic,23 hemostatic,31 antibacterial,28 and antiviral.32 For osteoarthritis and other musculoskeletal conditions, stinging nettle above ground parts might work due to potential analgesic and anti-inflammatory effects.24,25
Some researchers think that stinging nettle might be beneficial for allergic rhinitis due to quercetin content. Quercetin is thought to have anti-inflammatory and mast-cell stabilizing effects. It decreases histamine release from basophils and mast cells.33
Stinging nettle seems to also act as a diuretic. The leaf juice can increase urine output and slightly decrease systolic blood pressure and body weight in people with venous insufficiency.23,28 Because of these effects, some people use stinging nettle for urinary tract disorders, including urinary tract infections (UTIs) and kidney stones. Stinging nettle also seems to decrease blood pressure and heart rate.23,31
Adverse Reactions: Stinging nettle above ground parts is generally well-tolerated.
Interactions with Drugs: Lithium Warfarin (Coumadin)
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: Kidney disease.
Dosage/Administration: Dr Clare’s Blends: 1 gm per day
For osteoarthritis, people typically use crude stinging nettle leaf 9 grams daily.24
For allergic rhinitis, people typically use stinging nettle leaf extract 300 mg three times daily. However, in some cases, 300 mg up to seven times daily has been used.22
Dr Clare’s Comment Stinging nettle leaf has a long history of use. It was used primarily as a diuretic and laxative as early as the times of the Greek physicians Dioscorides and Galen.
Specific References: STINGING NETTLE 22. Mittman P. Randomized, double-blind study of freeze-dried Urtica dioica in the treatment of allergic rhinitis. Planta Med 1990;56:44-7. 23. Monographs on the medicinal uses of plant drugs. Exeter, UK: European Scientific Co-op Phytother, 1997. 24. Mills S, Bone K. Principles and Practice of Phytotherapy. London: Churchill Livingstone, 2000. 25. Randall C, Randall H, Dobbs F, et al. Randomized controlled trial of nettle sting for treatment of base-of-thumb pain. J R Soc Med 2000;93:305-9. 26. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 27. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. Stuttgart: Medpharm GmbH Scientific Publishers, 1994. 28. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. 29. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. 30. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 31. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 32. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 33. Anon. Quercetin. Alt Med Rev 1998;3:140-3. | |
P |
|---|
Pasque Flower
Easter Flower. Scientific Name: Anemone pulsatilla. Family: Ranunculacaeae. People Use This For: Pulsatilla is used for painful conditions of the male or female reproductive system, such as painful periods. Pulsatilla is also used for tension headache, hyperactive states, insomnia, migraines, neuralgia, general restlessness, diseases and functional disorders of the gastrointestinal GI and urinary tract. Safety: There are no information scientific studies available.
Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: There is insufficient reliable information available about the effectiveness of pulsatilla. Mechanism of Action: The applicable parts of pulsatilla are the above ground parts. Pulsatilla has analgesic, antispasmodic, sedative, and antibacterial properties. It exhibits both uterine stimulant and depressant activities. Pulsatilla contains ranunculin. Ranunculin hydrolyzes to a toxic unstable compound called protoanemonin, which readily dimerizes to nontoxic anemonin.84 Protoanemonin causes central nervous system (CNS) stimulation, then paralysis in experimental animals. It also has antimicrobial activity.85 Both anemonin and protoanemonin show some evidence of sedative and antipyretic activity.84 Irritation of the kidney and urinary tract might be due to the alkylating action of protoanemonin.85 Some evidence suggests anemonin might be cytotoxic.84 Interactions with Herbs & Supplements: None known. Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None known. Dosage/Administration: Dr Clare’s Blends: 1 gm/day. Oral: A typical oral dose is 120-300 mg dried above ground parts three times daily. Alternatively one cup tea consumed three times daily. To make tea, steep or simmer 120-300 mg dried above ground parts in 150 mL water 5-10 minutes, strain,84 0.12-0.3 mL of the liquid extract, 1:1 in 25% alcohol, has been used three times daily.84 0.3-1 mL of the tincture, 1:10 in 40% alcohol, has been used three times daily.84 Topical: No typical dosage. Specific References: PULSATILLA 84. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 85. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. | |
Passion Flower
Pasiflora, Passiflorae Herba. Scientific Name: Passiflora incarnata. Family: Passifloraceae. People Use This For: Passionflower is used for insomnia, gastrointestinal upset related to anxiety or nervousness, generalized anxiety disorder. Passionflower is also used orally for neuralgia, generalized seizures, spasmodic asthma, menopausal symptoms, attention deficit-hyperactivity disorder , nervousness and excitability, palpitations, heart rhythm abnormalities, high blood pressure, fibromyalgia, and pain relief. In foods and beverages, passionflower extract is used as a flavoring. Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Passionflower has Generally Recognized As Safe status (GRAS) for use in foods in the US.46 No concerns regarding safety when used orally and appropriately, short-term for medicinal purposes. There is evidence that passionflower liquid extracts can be safely used for up to one month.47,48,49 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: POSSIBLY EFFECTIVE Adjustment disorder with anxious mood. A specific combination product (Euphytose, EUP) that includes passionflower seems to decrease some symptoms of adjustment disorder with anxious mood. However, it's not known which ingredient or ingredients are responsible for the beneficial effects. Other herbs in the product are crataegus, ballota, and valerian, which have mild sedative effects, and cola and paullinia with stimulant properties.50 Anxiety. There is some evidence that passionflower can reduce symptoms of anxiety.51 Some research shows that a liquid extract 45 drops daily is comparable to oxazepam (Serax) 30 mg for treating symptoms of GAD in some patients.49 Additional research shows that a different passionflower extract 90 mg/day reduces symptoms of non-specific anxiety comparable to mexazolam.52 Opiate withdrawal. Passionflower liquid extract 60 drops, in combination with clonidine 0.8 mg daily, seems to be significantly better than clonidine alone when used for reducing symptoms such as anxiety, irritability, insomnia, and agitation. However, the combination is no better than clonidine alone for physical symptoms such as tremor and nausea.48 Mechanism of Action: The applicable parts of passionflower are the above ground parts. Passionflower contains several active constituents including a range of flavonoids. The harman (harmala) alkaloids identified in passionflower include harmine, harmaline, harmalol, harman, and harmin.53,54,55 Other constituents include maltol and ethyl maltol.55 Passionflower has sedative, hypnotic, anxiolytic, analgesic, and antispasmodic effects.53,54 Some evidence suggests the passionflower constituent apigenin binds to central benzodiazepine receptors,56 possibly causing anxiolytic effects without impairing memory or motor skills.56 Other evidence suggests passionflower extracts might reduce amphetamine-induced hypermotility, aggressiveness, and restlessness; and raise the pain threshold.57,58,59 Adverse Reactions: Passionflower is generally well tolerated with few side effects.R2pp.329Uncommonly Passionflower may cause dizziness, confusion, sedation, and unsteady gait.49,52,51 There are 2 case reports of possible severe side effects with Passiflora in the literature. Interactions with Herbs & Supplements: Herbs and Supplements with Sedative Properties: May enhance the effectiveness. Interactions with Drugs: CNS Depressants: May enhance the effectiveness. Refer to a Medical Herbalist or other Medical Adviser. Interactions with Foods: None known. Interactions with Lab Tests: None known. Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture. Oral: The average amount of passionflower is 4-8 grams per day. References: monograph: PASSIONFLOWER 46. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 47. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 48 Akhondzadeh S, Kashani L, Mobaseri M, et al. Passionflower in the treatment of opiates withdrawal: a double-blind randomized controlled trial. J Clin Pharm Ther 2001;25:369-73. 49. Akhondzadeh S, Naghavi HR, Shayeganpour A, et al. Passionflower in the treatment of generalized anxiety: a pilot double-blind randomized controlled trial with oxazepam. J Clin Pharm Ther 2001;26:363-7. 50. Bourin M, Bougerol T, Guitton B, Broutin E. A combination of plant extracts in the treatment of outpatients with adjustment disorder with anxious mood: controlled study vs placebo. Fundam Clin Pharmacol 1997;11:127-32. 51. Miyasaka LS, Atallah AN, Soares BG. Passiflora for anxiety disorder. Cochrane Database Syst Rev 2007;(1):CD004518. 52. Mori A, Hasegawa K, Murasaki M, et al. Clinical evaluation of Passiflamin (passiflora extract) on neurosis - multicenter double blind study in comparison with mexazolam. Rinsho Hyoka Clinical Evaluation) 1993;21:383-440. 53. Dhawan K, Kumar S, Sharma A. Anxiolytic activity of aerial and underground parts of Passiflora incarnata. Fitoterapia 2001;72:922-6. 54. Dhawan K, Kumar S, Sharma A. Anti-anxiety studies on extracts of Passiflora incarnata Linneaus. J Ethnopharmacol 2001;78:165-70. 55. Aoyagi N, Kimura R, Murata T. Studies on passiflora incarnata dry extract. I. Isolation of maltol and pharmacological action of maltol and ethyl maltol. Chem Pharm Bull 1974;22:1008-13. 56. Salgueiro JB, Ardenghi P, Dias M, et al. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effect on memory tasks in rats. Pharmacol Biochem Behav 1997;58:887-91. 57. Monographs on the medicinal uses of plant drugs. Exeter, UK: European Scientific Co-op Phytother, 1997. 58. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 59. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996.
| |
Peppermint
Scientific name: Mentha piperita. Botanical family: Lamiaceae/Labiatae. Part used: Leaves and stems. Traditional use. Peppermint is used for the common cold, cough, inflammation of the mouth and throat, sinusitis, runny nose, fever, liver and gallbladder complaints, irritable bowel syndrome, cramps of the upper gastrointestinal tract and bile ducts, dyspepsia, fever, flatulence, and for tension headache. It is also used for nausea, vomiting, morning sickness, respiratory infections, painful periods, diarrhea, small intestinal bacterial overgrowth, and as a stimulant. Inhaled peppermint oil is used for symptomatic treatment of cough and colds. Peppermint oil is used directly on the skin as an analgesic for pain.
Peppermint is a common flavoring agent in foods and beverages. Peppermint oil is used as a fragrance component in soaps and cosmetics, and as a flavoring agent in pharmaceuticals.
Safety. There are no concerns regarding safety when used in amounts commonly found in foods. Peppermint has Generally Recognized as Safe (GRAS) status in the US.1 There are no concerns regarding safety when used in medicinal amounts in children 8 years of age and older. Traditionally catmint is used for children as it is less stimulant than peppermint e.g. Dr Clare’s Childrens Tea Blend.
Pregnancy: No concerns regarding safety when used orally in amounts commonly found in foods. Peppermint has Generally Recognized as Safe (GRAS) status in the US.1 Breastfeeding: as for pregnancy. Constituents. Essential oils, containing menthol, menthone and menthyl acetate. Trace constituents include alpha-pinene, sabinene, terpinolene, ocimene, gamma-terpinene, fenchene, alpha- and beta-thujone, citronellol, and other compounds.2,3 Flavonoids; rutin, menthoside, luteolinand rutinosides Phenolic acids and lactones; rosmarinic acid, and others. Miscellaneous; azulenes, choline, carotenes etc
Scientific evidence. No clinical studies have been done. Mechanism of action. Activity is primarily, but not exclusively, due to the actions of the essential oils, from which a direct action on smooth muscle organs cause a stronger anti-spasmodic effect than some of its individual components. The antispasmodic effect has been confirmed on the small intestines of guinea pigs.8 Peppermint tea brings about a considerable increases in the production of bile. This activity has been confirmed in animal experiments with dogs. 9 The antispasmodic effect of peppermint oil is used internally for irritable bowel syndrome4 e.g. the medication colpermin which is derived from peppermint oil. This appears to result from direct relaxing effects on the gastrointestinal tract smooth muscle, characteristic of calcium antagonist action. Be careful with the oral use of any essential oils as they have very powerful effects. The oils have very concentrated constituents. Seek professional advice.
For pain in myalgias (muscle pain) and neuralgias (nerve pain), menthol in topical peppermint oil is thought to have a direct inhibitory effect on the sensitized pain receptors. Menthol might also act on the central nervous system to alter pain perception.5
Preliminary research suggests that luteolin-7-O-rutinoside from peppermint leaf can inhibit histamine release.2 Laboratory models of allergic rhinitis (runny nose) suggest that peppermint leaf extract might relieve nasal symptoms.6 A mild sedative action has been confirmed in animal experiments in mice.10
Adverse Reactions:
Topically; peppermint oil can cause skin irritation and contact dermatitis.5 Always patch test on normal skin before use. Contact sensitivity in the mouth has been reported.7
Interactions with herbs and supplements. None known.
Interactions with Drugs. avoid internal use of peppermint oil with Cyclosporine (Neoral, Sandimmune), which is an immune suppressant: None known.
Dosage. Recommended dose: 2-7mls per day 1:5 tincture 30% alcohol. Infusion: range from 3-6 tsps. per day. Powder/capsule: range from 2-4 gms per day. Liquid extract: 2-4ml/day. Dr Clare’s Joint Cleansing Tea provides ½ tsp. per day of peppermint.
References. 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 2. Inoue T, Sugimoto Y, Masuda H, Kamei C. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol Pharm Bull 2002;25:256-9. 3. Nair B. Final report on the safety assessment of Mentha Piperita (Peppermint) Oil, Mentha Piperita (Peppermint) Leaf Extract, Mentha Piperita (Peppermint) Leaf, and Mentha Piperita (Peppermint) Leaf Water. Int J Toxicol 2001;20:61-73. 4. Storr M, Sibaev A, Weiser D, et al. Herbal extracts modulate the amplitude and frequency of slow waves in circular smooth muscle of mouse small intestine. Digestion 2004;70:257-63 5. Davies SJ, Harding LM, Baranowski AP. A novel treatment of postherpetic neuralgia using peppermint oil. Clin J Pain 2002;18:200-2. 6. Inoue T, Sugimoto Y, Masuda H, Kamei C. Effects of peppermint (Mentha piperita L.) extracts on experimental allergic rhinitis in rats. Biol Pharm Bull 2001;24:92-5. 7. Morton CA, Garioch J, Todd P, et al. Contact sensitivity to menthol and peppermint in patients with intra-oral symptoms. Contact Dermatitis 1995;32:281-4. 8. Forster HB, Niklas H, Lutz S. Planta Med 40, 309-319 (1980). 9. Della Loggia R, Tibaro A, Lunder TL. Fitoterapia 61, 215-221 (1990). 10. Miething H, Holz W. Pharm. Ztg. 133. 16-17. (1988) | |
PlantainAlso Known As: Brown Psyllium, Dietary Fiber, Fibre Alimentaire, Fleaseed, Fleawort, French Psyllium, Graine de Psyllium, Herbe aux Puces, Œil-de-Chien, Plantain, Plantain Pucier, Psyllion, Psyllios, Psyllium, Psyllium Brun, Psyllium d'Espagne, Psyllium Noir, Psyllium Seed, Pucière, Pucilaire, Spanish Psyllium, Zaragatona. Scientific Name: Plantago psyllium, synonym Psyllium afra; Psyllium indica, synonym Psyllium arenaria. People Use This For: Orally, black psyllium is used for chronic constipation and for softening stools in conditions such as hemorrhoids, anal fissures, anorectal surgery, and pregnancy. It is also used orally for diarrhea, irritable bowel syndrome (IBS), reducing elevated cholesterol, dysentery, and treating cancer. Safety: LIKELY SAFE...when used orally with appropriate fluid intake (3,6,8). Effectiveness: Constipation. Taking black psyllium orally works as a bulk laxative and reduces constipation (8). Hypercholesterolemia. Taking black psyllium orally seems to reduce total and LDL cholesterol, and the LDL:HDL ratio (4,5). Mechanism of Action: The applicable part of black psyllium is the seed. Its constituents are not absorbed and have no systemic effects (1). Black psyllium seed forms a mucilaginous mass when mixed with water and has a bulk laxative effect (1,3,4). In people with diarrhea, the mucilage absorbs water, provides mass, and prolongs gastrointestinal transit (1,4). In individuals with constipation, the mucilage absorbs water, swells, and stimulates peristalsis, reducing gastrointestinal transit time (1,3,4). Black psyllium can decrease abdominal pain in people with irritable bowel syndrome (IBS) by reducing rectosigmoidal pressure (9). Psyllium reduces peak blood glucose levels by slowing carbohydrate absorption (1,4) and can decrease cholesterol by absorbing dietary fats in the gastrointestinal tract, thereby preventing systemic absorption. It can also increase cholesterol elimination in the fecal bile acids (1,4,5,6). Chewing or crushing the seeds can release a pigment that deposits in renal tubules (5) and can be nephrotoxic (4). This pigment is removed from most commercial products (4). Adverse Reactions: Orally, black psyllium can cause transient flatulence and abdominal distention (3). When consumed without water, it can cause esophageal (3) and bowel obstruction (3,12). Chewing or crushing the seeds can release a pigment that deposits in the renal tubules (5) and can be nephrotoxic (4). This pigment is removed from most commercial products (4). Allergic reactions to black psyllium include allergic rhinitis, conjunctivitis, urticaria, and asthma (7). Interactions with Herbs & Supplements: None known. Interactions with Drugs: ANTIDIABETES DRUGS <<interacts with>> BLACK PSYLLIUM Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Possible • Level of Evidence = B Black psyllium can reduce blood glucose levels in patients with type 2 diabetes (22) and might have additive effects on glucose levels when used with antidiabetes drugs. Monitor blood glucose levels closely. Medication dose adjustments may be necessary. Some antidiabetes drugs include glimepiride (Amaryl), glyburide (Diabeta, Glynase PresTab, Micronase), insulin, pioglitazone (Actos), rosiglitazone (Avandia), and others. CARBAMAZEPINE (Tegretol) <<interacts with>> BLACK PSYLLIUMInteraction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = D Black psyllium can reduce carbamazepine absorption (10). DIGOXIN (Lanoxin) <<interacts with>> BLACK PSYLLIUMInteraction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = D Concomitant use might reduce digoxin absorption (1), requiring dose adjustment (6). LITHIUM <<interacts with>> BLACK PSYLLIUMInteraction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = D Black psyllium use can reduce serum lithium levels (11). The fiber in lithium might decrease the absorption of lithium. Interactions with Foods: NUTRIENT ABSORPTION: The long-term use of black psyllium with meals can reduce nutrient absorption requiring vitamin or mineral supplementation (6). Interactions with Lab Tests: BLOOD GLUCOSE: Theoretically, black psyllium might lower postprandial blood glucose levels and test results (1,4,13). Interactions with Diseases or Conditions: DIABETES: Black psyllium can lower blood glucose levels in people with type 2 diabetes by retarding carbohydrate absorption (13,17). Monitor blood glucose levels closely. Doses of conventional antidiabetes medications may require adjustment. Also, warn patients with diabetes that some commercial blond psyllium products can contain added sugars and other absorbable carbohydrates which might increase blood glucose levels. GI CONDITIONS: Black psyllium is contraindicated in people with fecal impaction, GI atony (1), GI tract narrowing, and obstruction or conditions that can lead to obstruction, such as spastic bowel (1,2,3,6,7). Dosage/Administration: ORAL: As a laxative, the typical dose of black psyllium seed is 10-30 grams per day (2,7), in divided amounts. Mix 10 grams seed in 100 mL water, to be followed by at least 200 mL water (7). Avoid chewing or crushing the seeds which can release a pigment that deposits in renal tubules (5). Adequate fluid intake is necessary and should be at least 150 mL water for each 5 grams of drug. The FDA labeling recommends at least 8 ounces (a full glass) of water or other fluid with each dose. Taking this product without enough liquid can cause choking (6). Black psyllium should be taken 30-60 minutes after a meal or the administration of other drugs (8). Editor's Comments:Black psyllium is an aggressive-growing, perennial weed found throughout the world. The plant was spread with the colonization of the New World and was nicknamed "Englishman's foot" by the North American Indians. Specific References: Plantain 1 Monographs on the medicinal uses of plant drugs. Exeter, UK: European Scientific Co-op Phytother, 1997. 2 Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 3 Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 4 The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 5 Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. 6 McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 7 Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. 1st ed. Montvale, NJ: Medical Economics Company, Inc., 1998. 8 Covington TR, et al. Handbook of Nonprescription Drugs. 11th ed. Washington, DC: American Pharmaceutical Association, 1996. 9 Cook IJ, Irvine EJ, Campbell D, et al. Effect of dietary fiber on rectosigmoid motility in patients with irritable bowel syndrome: A controlled, crossover study. Gastroenterology 1990;98:66-72. 10 Etman M. Effect of a bulk forming laxative on the bioavailablility of carbamazepine in man. Drug Dev Ind Pharm 1995;21:1901-6. 11 Perlman BB. Interaction between lithium salts and ispaghula husk. Lancet 1990;335:416. 12 Agha FP, Nostrant TT, Fiddian-Green RG. Giant colonic bezoar: a medication bezoar due to psyllium seed husks. Am J Gastroenterol 1984;79:319-21. 13 Anderson JW, Allgood LD, Turner J, et al. Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hypercholesterolemia. Am J Clin Nutr 1999;70:466-73. 14 Suhonen R, Kantola I, Bjorksten F. Anaphylactic shock due to ingestion of psyllium laxative. Allergy 1983;38:363-5. 15 Vaswani SK, Hamilton RG, Valentine MD, Adkinson NF. Psyllium laxative induced anaphylaxis, asthma, and rhinitis. Allergy 1996;51:266-8. 16 Freeman GL. Psyllium hypersensitivity. Ann Allergy 1994;73:490-2. 17 Wolever TM, Vuksan V, Eshuis H, et al. Effect of method of administration of psyllium on glycemic response and carbohydrate digestibility. J Am Coll Nutr 1991;10:364-71. 18 Lantner RR, Espiritu BR, Zumerchik P, Tobin MC. Anaphylaxis following ingestion of a psyllium-containing cereal. JAMA 1990;264:2534-6. 19 Schneider RP. Perdiem causes esophageal impaction and bezoars. South Med J 1989;82:1449-50. 20 Shulman LM, Minagar A, Weiner WJ. Perdiem causing esophageal obstruction in Parkinson's disease. Neurology 1999;52:670-1. 21 Kaplan MJ. Anaphylactic reaction to "Heartwise." N Engl J Med 1990;323:1072-3. 22 Frati Munari AC, Benitez Pinto W, Raul Ariza Andraca C, Casarrubias M. Lowering glycemic index of food by acarbose and Plantago psyllium mucilage. Arch Med Res 1998;29:137-41. | |
Prickly Ash
Scientific name: Zanthoxylum americanum. Botanical Family: Rutaceae. Parts used: Bark. Traditional use. Northern prickly ash is used for muscle cramps, Raynaud's syndrome, chronic rheumatic conditions, poor peripheral circulation associated with rheumatic symptoms, intermittent claudication, leg ulcers associated with poor circulation, inflammation, as a stimulant tonic, for toothache, and as a sweating promoter in fever. Safety. There are no concerns regarding safety when the bark is used appropriately in medicinal amounts.1 Pregnancy: Consult a medical herbalist. Breastfeeding: Consult a medical herbalist. Constituents. Isoquinolone alkaloids; alpha fagarine, beta fagarine, magnoflorine, laurifoline, nitidine, chelerythrine, tambatarine and candicine. Coumarins; including xanthyletin, xanthoxyletin, xanthotoxin, alloxanthyletin, isoorientin, cnidilin, dipetalin, psorelan and imperatorin. Lignans; sesamin and asarinin. Resin, acid volatile oil, and tannins, which may be pharmacologically active. Clinical evidence. There are no clinical trials. Mechanism of action. There is woefully little research about the possible mechanism of action and active ingredients for this medicinal herb despite widespread use in many countries. Chelerythrine is antimicrobial. Zanthoxylum americanum demonstrates antifungal activity.3 Furanocoumarins found in berries of Zanthoxylum americanum (for example psoralen.) have demonstrated cytotoxic activity against human tumor cells.4 Adverse reactions. None reported. Interactions with drugs. None reported. Interactions with foods. None known. Interactions with laboratory tests. None known. Interactions with diseases or conditions. Gastrointestinal conditions. May increase stomach acid. None known. Dosage. Recommended dose: 1.5-5 mls per day 1:5 tincture 45% alcohol. Decoction: I tsp. per day. Raw bark: 1-3 grams three times daily; The recommended dose of Dr Clare’s Joint Support Blend provides 3mls per day of 1:3 Tincture in 15mls daily, at a dose of 5mls three times a day. This is equivalent to 375-750mgs per day. References: 1. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 2. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 3. Bafi-Yeboa, N. F. A., Arnason, J. T., Baker, J., Smith, M.L. (2005). Antifungal constituents of Northern prickly ash, Zanthoxylum americanum Mill. Phytomedicine, 28, 370-377. 4. Saquib, Q. N., Hui, Y.-H., Anderson, J. E., Mclaughlin, J. L. (1990). Bioactive Furanocoumarins from the Berries of Zanthoxylum americanum. Phytotherapy Research, 4, 216-219. ISSN: 1099-1573. | |
R |
|---|
Raspberry leaf (Red Raspberry)
Framboise, Framboise Rouge, Framboisier Rouge, Framboisier Sauvage, Frambuesa Roja, Raspberry, Rubi Idaei Folium, Rubus. Scientific Name: Rubus idaeus, synonym Rubus buschii, Rubus strigosus. People Use This For: Orally, red raspberry leaf is used for GI tract disorders, upper and lower respiratory tract disorders, cardiovascular system disorders, influenza, swine flu, fever, diabetes, vitamin deficiency, as a diaphoretic or diuretic, for stimulating bile production, "purification of skin and blood", diarrhea, dysmenorrhea, menorrhagia, morning sickness associated with pregnancy, preventing miscarriage, and facilitating labor and delivery. Topically, red raspberry leaf is used for inflammation of the mouth and throat, and skin rash and inflammation. Safety: LIKELY SAFE ...when used orally in amounts commonly found in foods (9). Effectiveness: Labor facilitation. Taking red raspberry leaf orally does not seem to reduce the length of labor or decrease the need for analgesics in the perinatal time period (5). There is insufficient reliable information available about the effectiveness of red raspberry leaf for its other uses. Machanism of Action: The applicable parts of red raspberry are the fruit (berry) and leaf. Red raspberry contains anthocyanidins, ellagitannins, flavonols such as quercetin and kaempferol, catechins, and phenolic acids. Other constituents include ascorbic acid, beta-carotene, chlorogenic acid, glutathione, and alpha-tocopherol (7,8 ,6). The contents of the fruit and leaves vary with maturity (8). Adverse Reactions: None reported. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: HORMONE SENSITIVE CANCERS/CONDITIONS: Red raspberry leaf might have estrogenic effects (3). Women with hormone sensitive conditions should avoid red raspberry leaf including breast, uterine, and ovarian cancer, endometriosis, and uterine fibroids. Dosage/Administration: ORAL: For facilitating labor, midwives typically prescribe red raspberry leaf tea prepared by steeping 2 grams dried leaf in 240 mL of boiling water for 5 minutes and then straining (2). Red raspberry leaf tablets 2.4 grams per day have been used for reducing labor pains, starting at 32 weeks gestation through labor (5). Editor's Comments: Red raspberry leaf extract has been used in Europe for centuries. The therapeutic use of red raspberry leaf was first described in 1597 in The Herbal, or a General History of Plants (1). Specific References: Raspberry
1. Eagon PK, Elm MS, Hunter DS, et al. Medicinal herbs: modulation of estrogen action. Era of Hope Mtg, Dept Defense; Breast Cancer Res Prog, Atlanta, GA 2000;Jun 8-11. 2. Parsons M, Simpson M, Ponton T. Raspberry leaf and its effects on labour: safety and efficacy. Aust Coll Midwives Inc J 1999;12:20-5. 3. Simpson M, Parsons M, Greenwood J, Wade K. Raspberry leaf in pregnancy: its safety and efficacy in labor. J Midwifery Womens Health 2001;46:51-9. 4. Wada L, Ou B. Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem 2002;50:3495-500. 5. Wang SY, Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem 2000;48:5677-84. 6. Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem 2000;48:140-6. 7. Mullen W, McGinn J, Lean ME, et al. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J Agric Food Chem 2002;50:5191-6. 8. Morimoto C, Satoh Y, Hara M, et al. Anti-obese action of raspberry ketone. Life Sci 2005;77:194-204. | |
Red Clover
Scientific name:Trifolium pratense. Family: Fabaceae/Leguminosae. Parts used: Flowering tops. Traditional use. Red clover is used for for its alterative action. It is anti-spasmodic, expectorant, sedative and acts on skin disorders. It is used for menopausal symptoms and hot flashes, cyclic breast pain or tenderness, premenstrual syndrome and for promotion of lymph flow. Red clover is used as a flavoring ingredient In drinks and foods. Safety. No concerns regarding safety when used orally in food amounts. Red clover has Generally Recognized As Safe (GRAS) status for use in foods in the US.1.2.3 There are no concerns regarding safety when used in traditional medicinal amounts. There is no evidence for any concerns when Red clover extracts have been used for up to one year . 4,5,6,7,8,9 Pregnancy: Refer to a medical herbalist. Breastfeeding: Refer to a medical herbalist. Constituents. Isoflavones; afrormosan, biochanin A, daidzein, formononetin, genistein, pratensein, calyconin, pseudobaptigin, orobol.irilone, and trifoside, and their glycoside conjugates. Other flavonoids, including pectolinarin and trifolin. Coumarins;coumestrol,medicagol and coumarin. Volatile oil;furfural. Miscellaneous; clovamides, L-dopa-caffeic acid conjugates, minerals, vitamins and phytoalexins. Scientific evidence. Breast Pain. Preliminary evidence suggests that red clover might relieve menstrual cycle breast pain. Red clover isoflavones 40-80 mg daily seem to reduce breast pain and tenderness in about 45% of patients.10 Menopausal symptoms. There is conflicting evidence about the effects of red clover on menopausal symptoms. 6,9,11,12,13,14,15 Mechanism of action. The flowering tops contain more than 100 different chemicals. Red clover contains phytoestrogens (plant estrogens), which are structurally similar to estrogens. 4,16,17,18 The health effects of methylated isoflavones have not been evaluated.19 Similar to isoflavones from soy, red clover isoflavones might act as selective estrogen-receptor modulators.16,20 In premenopausal women with normal endogenous estrogen levels, isoflavones may have an anti-estrogen effect. In postmenopausal women with low endogenous estrogens, isoflavones are likely to act as weak estrogens. 21,22,23,24,25,26 It is suggested that red clover might have anti-anxiety effects due to its beta estrogen receptor agonist activity.9 Red clover is thought to be beneficial for preventing osteoporosis due to its weak estrogenic effects . 5,16,26. The isoflavones also appears to directly inhibit the breakdown of bone.19 Red clover isoflavones don't seem to lower cholesterol. 4,7,8 Some researchers still think that red clover might play a role in improving cardiovascular health by increasing bile acid excretion, up-regulating low-density lipoprotein (LDL) receptors, and lowering resistance in the systemic arterial blood vessels. 19,26,27,28,29. Adverse reactions. Red clover is generally well tolerated. 6,7,8,9 It can rarely cause rash-like reactions, muscle pain, headache, nausea, and vaginal spotting.30 There is some concern that red clover might increase the risk of endometrial hyperplasia due to its potential estrogenic effects. However, the ingestion of phytoestrogens in dietary amounts, 1-3 mg isoflavones per day, does not seem to increase the risk of endometrial cancer.3 Preliminary evidence also suggests that taking red clover isoflavones 50 mg daily does not have any significant effect on endometrial growth in women age 45-53 when taken for 3 months.18 Interactions with herbs and supplements. None reported. Interactions with drugs: Tamoxifen (breast cancer treatment): Always consult an Herbal Medicine Physician. Interactions with foods: None known. Interactions with laboratory tests: None known. Interactions with diseases or conditions: Breast Cancer: Refer to an Herbal Medicine Physician. None known. Dosage. Recommended dose: 4-15mls per day 1:5 tincture 30% alcohol. Insfusion: range from 2-6 tsps. per day. Powder/capsule: 750mgs per day. Raw herb: 4gms per day. Liquid extract: 2-4mls/day. Dr Clare’s Joint Cleansing Tea provides ½ tsp. per day of peppermint. References. 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. [Accessed 22/6/2014] 2. Nelsen J, Barrette E, Tsouronix C, et al. Red clover (Trifolium pratense) monograph: A clinical decision support tool. J Herb Pharmacother. 2002;2:49-72. 3. Horn-Ross PL, John EM, Canchola AJ, et al. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst 2003;95:1158-64. 4. Howes JB, Sullivan D, Lai N, et al. The effects of dietary supplementation with isoflavones from red clover on the lipoprotein profiles of postmenopausal women with mild to moderate hypercholesterolemia. Atherosclerosis 2000;152:143-7. 5. Atkinson C, Compston JE, Day NE, et al. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2004;79:326-33. 6. van de Weijer P, Barentsen R. Isoflavones from red clover (Promensil) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas 2002;42:187-93. 7. Schult TM, Ensrud KE, Blackwell T, et al. Effect of isoflavones on lipids and bone turnover markers in menopausal women. Maturitas 2004;48:209-18. 8. Atkinson C, Oosthuizen W, Scollen S, et al. Modest protective effects of isoflavones from a red clover-derived dietary supplement on cardiovascular disease risk factors in perimenopausal women, and evidence of an interaction with ApoE genotype in 49-65 year-old women. J Nutr 2004;134:1759-64. 9. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause 2009;16:1156-66. 10. Ingram DM, Hickling C, West L, et al. A double-blind randomized controlled trial of isoflavones in the treatment of cyclical mastalgia. The Breast 2002;11:170-4. 11. Baber RJ, Templeman C, Morton T, et al. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999;2:85-92. 12. Knight DC, Howes JB, Eden JA. The effect of Promensil, an isoflavone extract, on menopausal symptoms. Climacteric 1999;2:79-84. 13. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006;295:2057-71. 14. Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol 2004;104:824-36. 15. Lipovac M, Chedraui P, Gruenhut C, et al. Improvement of postmenopausal depressive and anxiety symptoms after treatment with isoflavones derived from red clover extracts. Maturitas 2010;65:258-61. 16. Umland EM, Cauffield JS, Kirk JK, et al. Phytoestrogens as therapeutic alternatives to traditional hormone replacement in postmenopausal women. Pharmacotherapy 2000;20:981-90. 17. Roberts DW, Doerge DR, Churchwell MI, et al. Inhibition of extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover). J Agric Food Chem 2004;52:6623-32. 18. Hale GE, Hughes CL, Robboy SJ, et al. A doubleblind randomized study on the effects of red clover isoflavones on the endometrium. Menopause 2001;8:338-46. 19. Anon. The role of isoflavones in menopausal health: consensus opinion of the North American Menopause Society. Menopause 2000;7:215-29. 20. This P, De La Rochefordiere A, Clough K, et al. Phytoestrogens after breast cancer. Endocr Relat Cancer 2001;8:129-34. 21. Zand RS, Jenkins DJ, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res Treat 2000;62:35-49. 22. Baird DD, Umbach DM, Lansdell L, et al. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab 1995;80:1685-90. 23. Duncan AM, Underhill KE, Xu X, et al. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab 1999;84:3479-84. 24. Ginsburg J, Prelevic GM. Lack of significant hormonal effects and controlled trials of phyto-oestrogens. Lancet 2000;355:163-4. 25. Hargreaves DF, Potten CS, Harding C, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab 1999;84:4017-24. 26. Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr 1999;129:758S-67S. 27. Nestel PJ, Pomeroy S, Kay S, et al. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J Clin Endocrinol Metab 1999;84:895-8. 28. Lissin LW, Cooke JP. Phytoestrogens and cardiovascular health. J Am Coll Cardiol 2000;35:1403-10. 29. Hodgson JM, Puddey IB, Beilin LJ, et al. Supplementation with isoflavonoid phytoestrogens does not alter serum lipid concentrations: a randomized controlled trial in humans. J Nutr 1998;128:728-32. 30. Tice J, Cummings SR, Ettinger B, et al. Few adverse effects of two red clover extracts rich in phytoestrogens: a multicenter, placebo-controlled trial. Alt Ther 2001;7:S33. | |
Rose hips
Apothecary Rose, Cynorhodon, Cynorhodons, Cynosbatos, Dog Rose, Dog Rose Hips, Églantier, Fruit de l'Églantier, Gulab, Heps, Hip, Hip Fruit, Hip Sweet, Hipberry, Hop Fruit, Persian Rose, Phool Gulab, Pink Rose, Poire d'oiseaux, Rosa de Castillo, Rosa Mosqueta, Rosae Pseudofructus Cum Semen, Rosehip, Rosehips, Rose Hips, Satapatri, Rose des Apothicaires, Rose de Provins, Rose Rouge de Lancaster, Rosier de Provence, Satapatrika, Shatpari, Wild Boar Fruit. CAUTION: See separate listings for Acerola, Vitamin C, and Cherokee Rosehip. Scientific Name: Rosa canina, synonym Rosa lutetiana; Rosa alba; Rosa centifolia; Rosa damascena; Rosa gallica, synonym Rosa provincialis; Rosa rugosa; Rosa villosa, synonym Rosa pomifera; other Rosa species. People Use This For: Orally, rose hip is used as a supplemental source of dietary vitamin C, for preventing and treating colds, influenza-like infections, infectious diseases, vitamin C deficiencies, fever, increasing immune function during exhaustion, gastric spasms, gastric acid deficiency, preventing gastric mucosal inflammation and gastric ulcers, and as a "stomach tonic" for intestinal diseases. It is also used orally for diarrhea, gallstones, gallbladder ailments, lower urinary tract and kidney disorders, dropsy (edema), gout, aging skin, disorders of uric acid metabolism, arthritis, sciatica, diabetes, increasing peripheral circulation, for reducing thirst, as a laxative and diuretic, and to treat chest ailments. Safety: LIKELY SAFE ...when used orally and appropriately. Rose hip extract has Generally Recognized as Safe (GRAS) status in the US (4912). A specific formulation of rose hip powder (LitoZin/i-flex, HybenVital, Denmark) taken in doses of 2.5 grams (5 capsules) twice daily has been safely used for 6 months (17416). Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Rheumatoid arthritis. Preliminary clinical research shows that taking a specific rose hip powder product (LitoZin/i-flex, HybenVital, Denmark) 2.5 grams (5 capsules) twice daily over 6 months improves patient scores on a disability index as well as other subjective physician- and patient-based assessments (17416). More evidence is needed to rate rose hip for this use. Mechanism of Action: Rose hip contains pectin, citric acid, and malic acid, which can have laxative and diuretic activities (5,18). The diuretic activity is controversial (8). Fresh rose hip contains between 0.5-1.7% vitamin C (5,8) and is estimated to contain 1250 mg vitamin C per 100 grams of rose hip (6). However, much of the vitamin C is destroyed during drying and processing (11), and declines rapidly with storage (2). Vitamin C is required for collagen formation and tissue repair (15). It is an enzyme cofactor in the synthesis of collagen, carnitine, norepinephrine, and peptide hormones, and in tyrosine metabolism (3042). Vitamin C is also involved in oxidation-reduction reactions, conversion of folic acid to folinic acid, carbohydrate metabolism, synthesis of lipids and proteins, iron metabolism, resistance to infections, and cellular respiration (15). It acts as an antioxidant, decreasing oxidants in gastric juice, decreasing lipid peroxidation, and decreasing oxidative DNA and protein damage (3042). Vitamin C deficiency that lasts for three to five months results in symptomatic scurvy that affects collagenous structures, bones, and blood vessels (15). Vitamin C enhances the absorption of soluble non-heme iron, either by reducing it (converting ferric to ferrous) or by preventing chelation by phytates or other food ligands (3042). Adverse Reactions: Orally, the adverse effects of vitamin C are related to the amount of vitamin C actually contained in the rose hip product. The adverse reactions include nausea; vomiting; esophagitis; heartburn; abdominal cramps; GI obstruction; fatigue; flushing; headache; insomnia; sleepiness; diarrhea; hyperoxaluria; and precipitation of urate, oxalate, or cysteine stones or drugs in the urinary tract (15). Large amounts are associated with deep vein thrombosis (15). The inhalation of the rose hip dust is reportedly a respiratory allergen in production workers. It can cause mild to moderate anaphylaxis (6). Topically, the rose hip dust ("itching powder") can cause itching by mechanical irritation (6). Interactions with Herbs & Supplements: IRON: Concomitant use interacts with the vitamin C in rose hip; 200 mg of vitamin C per 30 mg of elemental iron increases oral iron absorption, especially ferric iron (3042). Interactions with Drugs: ALUMINUM <<interacts with>> ROSE HIP Interaction Rating = Moderate Be cautious with this combination. Severity = Mild • Occurrence = Probable • Level of Evidence = D Concomitant use interacts with the vitamin C in rose hip and can increase aluminum absorption, but the clinical significance of this is unknown (3046). Administer rose hip with vitamin C two hours before or four hours after antacids (3046). ASPIRIN <<interacts with>> ROSE HIP Interaction Rating = Minor Be watchful with this combination. Severity = Mild • Occurrence = Possible • Level of Evidence = D The vitamin C in rose hip can increase urinary excretion of ascorbic acid and decrease excretion of salicylates, but this may not have a clinically significant effect on salicylate plasma levels (3046). CHOLINE MAGNESIUM TRISALICYLATE (Trilisate) <<interacts with>> ROSE HIP Interaction Rating = Minor Be watchful with this combination. Severity = Mild • Occurrence = Possible • Level of Evidence = D The vitamin C in rose hip can increase urinary excretion of ascorbic acid and decrease excretion of salicylates such as choline magnesium trisalicylate. But this may not have a clinically significant effect on salicylate plasma levels (3046). ESTROGENS <<interacts with>> ROSE HIP Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Possible • Level of Evidence = D Theoretically, the vitamin C in large amounts of Cherokee rosehip might increase absorption and effects of estrogen (129,130). FLUPHENAZINE (Prolixin) <<interacts with>> ROSE HIP Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = D Concomitant use with rose hip decreases blood levels due to vitamin C content (15). LITHIUM <<interacts with>> ROSE HIP Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate • Occurrence = Probable • Level of Evidence = D Rose hip is thought to have diuretic properties. Theoretically, due to these potential diuretic effects, rose hip might reduce excretion and increase levels of lithium. The dose of lithium might need to be decreased. SALSALATE (Disalcid) <<interacts with>> ROSE HIP Interaction Rating = Minor Be watchful with this combination. Severity = Mild • Occurrence = Possible • Level of Evidence = D The vitamin C in rose hip can increase urinary excretion of ascorbic acid and decrease excretion of salicylates such as salsalate. But this may not have a clinically significant effect on salicylate plasma levels (3046). WARFARIN (Coumadin) <<interacts with>> ROSE HIP Interaction Rating = Moderate Be cautious with this combination. Severity = High • Occurrence = Possible • Level of Evidence = D Concomitant use interacts with the vitamin C in rose hip. Large amounts of vitamin C can impair the warfarin response (3046). Interactions with Foods: IRON: Concomitant use interacts with the vitamin C in rose hip and can increase the absorption of dietary (ferric) iron (3042). Interactions with Lab Tests: ACETAMINOPHEN: The vitamin C in rose hip can cause false negative urine results with methods based on hydrolysis and formation of an indophenol blue chromagen (275). LDH: The vitamin C in rose hip can cause a false decrease measured by Technicon SMA 12/60 and Abbott 100 methods (275). Interactions with Diseases or Conditions: DIABETES: The vitamin C in rose hip might affect glycogenolysis and the control of diabetes, but not all experts agree on this (15). Dosage/Administration: ORAL: The typical dose of rose hips is as a tea, which is prepared by steeping 2-2.5 grams of the crushed rose hips in 150 mL boiling water for 10-15 minutes and then straining(8). For rheumatoid arthritis, a specific rose hip powder product (LitoZin/i-flex, HybenVital, Denmark) 2.5 grams (5 capsules) twice daily has been used (17416). Editor's Comments: Rose hip with seed is the ripe, dried receptacle (hip) with fruit (seed) of various Rosa species, including dog rose (Rosa canina), white rose (Rosa alba), provence rose (Rosa centifolia), and damask rose (Rosa damascena). Avoid confusion with Cherokee rosehip, rose flower, and vitamin C. CAUTION: Sometimes rose hip seeds or plain rose hip receptacles without seeds are sold. These are different than rose hip with seed. Fresh rose hips contain a high concentration of vitamin C; however, much of the vitamin C is destroyed during drying and processing (11) and declines rapidly with storage (2). Many rose hip-derived "natural" vitamin C products are supplemented with synthetic vitamin C (6,11), but may not be clearly labeled accordingly (6). Specific References: Rose Hips
| |||||||||||||||||||||||||||||||||||||||
Rosemary
Scientific Name: People Use This For: Safety: Effectiveness: Mechanism of Action: Adverse Reactions: Interactions with Herbs & Supplements: Interactions with Drugs: Interactions with Foods: Interactions with Lab Tests: Interactions with Diseases or Conditions: Dosage/Administration: Specific References: Rosemary
| |
S |
|---|
Sage
Common Sage, Garden Sage, Meadow Sage. Scientific Name: Salvia officinalis. Family: Lamiaceae/Labiatae. People Use This For: Orally, sage is used for loss of appetite; excessive perspiration; painful periods; diarrhea; gastritis;reducing discharge from the breast; reduction of saliva secretion; and digestive problems including flatulence, bloating, and dyspepsia. It is also used for depression, cerebral ischemia, memory enhancement, and Alzheimer's disease. Topically, sage is used for herpes labialis, laryngitis, pharyngitis, stomatitis, gingivitis, glossitis, minor oral injuries, and inflammation of the nasal mucosa. In foods, it is used as a culinary spice. In manufacturing, sage is used as a fragrance component in soaps and cosmetics. Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Sage is approved for food use in the US.13 No concerns regarding safety when used orally in therapeutic doses, short term. Sage seems to be safe when taken orally for up to 4 months.140,141 No concerns regarding safety when used topically.142 Pregnancy and Lactation: Traditionally used to dry up breast feeding. Avoid in breast feeding mothers. Effectiveness: POSSIBLY EFFECTIVE Alzheimer's disease. Taking extracts of Salvia officinalis and Salvia lavandulaefolia orally seem to improve cognitive function in patients with mild to moderate Alzheimer's disease when used for up to 4 months.140,141 Herpes labialis (cold sores). Topical treatment of herpes labialis with a cream containing sage and rhubarb (Rheum officinale and Rheum palmatum) may be about as effective as acyclovir (Zovirax) cream. Acyclovir cream provides healing of lesions in 6.3 days; the sage and rhubarb cream provides healing of lesion in 7.2 days. The combination of sage and rhubarb appears to improve the time to healing and to reduce pain, compared with sage alone.142 INSUFFICIENT RELIABLE EVIDENCE to RATE Memory. Single doses of Salvia lavandulaefolia might enhance memory in a dose-dependent manner in young adults.143 More evidence is needed to rate sage for this use. Mechanism of Action: The applicable part of sage is the leaf and volatile oil. Adverse Reactions: Orally, sage can cause nausea, vomiting, abdominal pain, dizziness, agitation.141 Interactions with Herbs & Supplements: None reported Interactions with Drugs: None reported. Interactions with Foods: None known. Dosage/Administration: Dr Clare’s Blends: 1 gm/day Usual dose: 1-12 gms /day in divided doses. Comments: Sage is a rich source of beta-carotene.144 Specific References: SAGE 139. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 140. Perry NS, Bollen C, Perry EK, Ballard C. Salvia for dementia therapy: review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav 2003;75:651-9. 141. Akhondzadeh S, Noroozian M, Mohammadi M, et al. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther 2003;28:47-9. 142. Saller R, Buechi S, Meyrat R, Schmidhauser C. Combined herbal preparation for topical treatment of Herpes labialis. Forsch Komplementarmed Klass Naturheilkd 2001;8:373-82. 143. Tildesley NT, Kennedy DO, Perry EK, et al. Salvia lavandulaefolia (Spanish Sage) enhances memory in healthy young volunteers. Pharmacol Biochem Behav 2003;75:669-74. 144. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. | |
Sarsaparilla
Smilax. Scientific Name: Smilax ornata; Smilax officinalis. Family: Smilacaceae. People Use This For: Sarsaparilla is used for psoriasis and other skin diseases, sweat inducer. Sarsaparilla is also used as an adjunct for treating leprosy and for syphilis. Mexican and Honduran sarsaparilla are used for treating gonorrhea, fevers, and digestive disorders. In manufacturing, sarsaparilla is used as a flavoring agent in foods, beverages, and pharmaceuticals. Mechanism of Action: The applicable part of sarsaparilla is the root. Sarsaparilla is thought to have antirheumatic, antiseptic, and antipruritic activity;52 however, these effects have not been substantiated. Sarsaparilla contains about 2% saponins and other varied constituents, including quercetin and phytosterols. The saponin constituents53,52,54,55 may have diuretic, sweat inducing, expectorant, and laxative effects.54 Sarsaparilla may improve appetite and digestion52 and its extracts may improve psoriasis symptoms.52 Preliminary evidence suggests that sarsaparilla may have hepato-protective and anti-inflammatory activity.52 Adverse Reactions: None reported in therapeutic doses. Interactions with Herbs & Supplements: None reported. Interactions with Drugs: None reported. Interactions with Foods: None known. Interactions with Lab Tests: None known. Dosage/Administration: Dr Clare’s Blends: 1 gm per day. Oral: The typical oral dose of sarsaparilla is 1-4 grams of the dried root or one cup of the tea three times daily.52 Specific References: SARSAPARILLA 50. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 51. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 52. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 53. Tyler VE. Herbs of Choice. Binghamton, NY: Pharmaceutical Products Press, 1994. 54. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 55. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Sarsaparilla has Generally Recognized As Safe status (GRAS) for use in foods in the US.50 No concerns regarding safety when used orally and appropriately for medicinal purposes51 no evidence of harm. Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available about the effectiveness of sarsaparilla. | |
Shepherd’s Purse
Scientific Name: People Use This For: Safety: Effectiveness: Mechanism of Action: Adverse Reactions: Interactions with Herbs & Supplements: Interactions with Drugs: Interactions with Foods: Interactions with Lab Tests: Interactions with Diseases or Conditions: Dosage/Administration: Specific References: Shepherd’s Purse | |
Siberian Ginseng
Eleuthero Ginseng, Eleutherococcus, Éleuthérocoque, Ginseng, Scientific Name: Eleutherococcus senticosus. Family: Araliaceae. People Use This For: Siberian ginseng is used as an adaptogen, for increasing resistance to environmental stress. It is also used orally for normalizing high or low blood pressure, insomnia, and increasing work capacity, chronic fatigue syndrome, diabetes, fibromyalgia, rheumatoid arthritis, influenza, swine flu, chronic bronchitis, improving athletic performance, reducing toxicity of chemotherapy, and symptomatic treatment of herpes simplex type II infections. It is also used orally as a general stimulant, diuretic, appetite stimulant, immune system stimulant, and for preventing colds and flu. Safety: No concerns regarding safety when used orally and appropriately, short-term. Siberian ginseng root extract has been used safely in clinical trials lasting up to 2 months.34,35,36,37,38,39 A specific combination product containing Siberian ginseng plus andrographis (Kan Jang, Swedish Herbal Institute) has also been safely used in multiple short-term clinical trials lasting 4-7 days.40,41,42,43,44,45,46 One clinical trial used this combination product in low doses for up to 3 months.47 There is insufficient scientific information available about the safety of Siberian ginseng when used long-term. There is no clinical evidence of adverse effects. Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: POSSIBLY EFFECTIVE Common cold. Some clinical research shows that taking a specific combination product containing Siberian ginseng plus andrographis (Kan Jang, Swedish Herbal Institute) orally significantly improves symptoms of the common cold when started within 72 hours of symptom onset. Some symptoms can improve after 2 days of treatment. It typically takes 4-5 days of treatment before there is maximal symptom relief.40,42,43,48,45,49 Some research suggests this combination of Siberian ginseng and andrographis relieves cold symptoms better than Echinacea or placebo in children.50 Herpes simplex virus type 2 (HSV-2). Taking a specific Siberian ginseng extract, standardized to contain eleutheroside 0.3% (Elagen), orally seems to reduce the frequency, severity, and duration of herpes simplex type II infections.34,35 POSSIBLY INEFFECTIVE Athletic performance. Taking Siberian ginseng orally doesn't seem to increase speed, quality, and capacity for physical work. A specific Siberian ginseng root liquid extract standardized to eleutherosides B and E content 3.4 mL daily doesn't seem to have any effect on endurance; performance; or psychological, cardiac, or respiratory parameters in trained distance runners.37,51 In trained endurance cyclists, Siberian ginseng 1200 mg per day (Endurox) doesn't seem to have any effect on glycogen, fat utilization, or cycling performance time.52 It also doesn't seem to improve respiration; reduce lactate production; or hasten heart rate recovery during stair-stepping exercise, treadmill, or cyclic ergometry.53,54,55 INSUFFICIENT SCIENTIFIC EVIDENCE to RATE Chronic fatigue syndrome. Taking Siberian ginseng orally 2 grams/day for 2 months does not reduce symptoms of chronic fatigue syndrome to the extent of clinical significance.38 Cognitive performance. There is preliminary evidence that suggests Siberian ginseng might improve memory and feelings of well-being in middle-aged people.36 Familial Mediterranean fever. Preliminary clinical research suggests that a combination of Siberian ginseng, andrographis, schisandra, and licorice (ImmunoGuard, Inspired Nutritionals) reduces the duration, frequency, and severity of attacks of familial Mediterranean fever in children.56 Heart disease. There is preliminary evidence that suggests administering Siberian ginseng intravenously might be useful for hyperlipidemia, and arrhythmias.57,58 Influenza. Preliminary clinical research suggests that patients with influenza who take a specific combination product containing Siberian ginseng plus andrographis (Kan Jang, Swedish Herbal Institute) have symptom relief more quickly compared to patients taking amantadine. Patients who take this combination also seem to have a reduced risk of post-influenza complication such as sinusitis or bronchitis.44 Ischemic stroke. There is preliminary evidence that suggests administering Siberian ginseng intravenously might be useful for treating acute cerebral infarction.59 Quality of life. Preliminary clinical research shows that Siberian ginseng significantly improves social functioning and mental well-being in people over 65 years of age after 4 weeks of treatment; however, this was not maintained after 8 weeks of treatment.39 More evidence is needed to rate Siberian ginseng for these uses. Mechanism of Action: The applicable parts of Siberian ginseng are the root and leaf. The root, which is most commonly used, contains active compounds referred to as eleutherosides A through M.60 Eleutheroside B (syringin) and eleutheroside E (syringaresinol) are the most plentiful and are used as marker compounds for Siberian ginseng products.61 The eleutherosides include a variety of diverse compounds including saponins (daucosterol, beta-sitosterol, hederasaponin B), coumarins (isofraxidin), lignans (sesamin, syringaresinol), phenylpropanoids (syringin, caffeic acid, sinapyl alcohol, coniferyl aldehyde, protocatechuic acid), betulinic acid, vitamin E, and provitamins like beta-carotene.60 Several constituents including syringin, syringoresinol, sesamin, beta sitosterol, caffeic acid, and coniferyl aldehyde are thought to have antioxidant and possible anticancer effects.60 In addition, there is some evidence that the constituent coniferyl aldehyde protects DNA against breakage caused by ultraviolet light.60 Siberian ginseng root extracts seem to have an antiproliferative effect on leukemia cells and appear to potentiate the effect of antimetabolites such as cytarabine.62 There's also preliminary evidence that suggests it might act as an antioxidant and prevent damage in ischemic stroke.59 Siberian ginseng root, the lignan constituent sesamin, and the phenylpropanoid syringin seem to have immunostimulatory effects.60 Siberian ginseng increases lymphocyte counts and phagocyte activity.63 Some eleutherosides have structural similarities to cardiac glycosides and can interfere with measurement of serum digoxin levels by some assay methods.64 There is no clinical evidence that Siberian ginseng has any of the pharmacological effects of cardiac glycosides. Siberian ginseng constituents have a variety of other pharmacological effects. Other constituents are also thought to be anti-inflammatory, sedative, diuretic, gonadotropic, estrogenic, protein-anabolic, and stimulate the pituitary-adrenocortical system.65 Siberian ginseng root extract seems to inhibit RNA-type viruses including human rhinovirus, respiratory syncytial virus (RSV), and influenza A virus, but has no effect on DNA viruses such as adenovirus or herpes simplex type 1 virus (HSV-1).66 Polysaccharide derivatives of Siberian ginseng appear to inhibit growth of the tuberculosis bacterium.67 Adverse Reactions: No adverse effects are expected if used as recommended.68 Interactions with Drugs: Digoxin (Lanoxin). Lithium. Interactions with Foods: None known. Interactions with Lab Tests: Digoxin Serum Assay: Siberian ginseng may interfere with some serum digoxin measurements. Dosage/Administration: Dr Clare’s Blend: 1 gm per day. 1-4gms daily Dr Clare’s Comment. An "Adaptogen" is a non-medical term used to suggest that a substance can act to strengthen the body and increase general resistance. It is often used to describe the activity of Siberian ginseng.60 Siberian ginseng is a completely different herb than American or Panax ginseng. Specific References: GINSENG, SIBERIAN 34. Williams M. Immuno-protection against herpes simplex type II infection by eleutherococcus root extract. Int J Altern Complem Med 1995;13:9-12. 35. Vogler BK, Pittler MH, Ernst E. The efficacy of ginseng. A systemic review of randomized clinical trials. Eur J Clin Pharmacol 1999;55:567-75. 36. Winther K, Ranlov C, Rein E, et al. Russian root (Siberian ginseng) improves cognitive functions in middle-aged people, whereas Ginkgo biloba seems effective only in the elderly. J Neurological Sci 1997;150:S90. 37. Dowling EA, Redondo DR, Branch JD, et al. Effect of Eleutherococcus senticosus on submaximal and maximal exercise performance. Med Sci Sports Exerc 1996;28:482-9. 38. Hartz AJ, Bentler S, Noyes R et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med 2004;34:51-61. 39. Cicero AF, Derosa G, Brillante R, et al. Effects of Siberian ginseng (Eleutherococcus senticosus maxim.) on elderly quality of life: a randomized clinical trial. Arch Gerontol Geriatr Suppl 2004;9:69-73. 40. Caceres DD, Hancke JL, Burgos RA, et al. Use of visual analogue scale measurements (VAS) to assess the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized, double-blind, placebo study. Phytomedicine 1999;6:217-23. 41. Thamlikitkul V, Dechatiwongse T, Theerapong S, et al. Efficacy of Andrographis paniculata, Nees for pharyngotonsillitis in adults. J Med Assoc Thai 1991;74:437-42. 42. Melchior J, Palm S, Wikman G. Controlled clinical study of standardized Andrographis paniculata in common cold- a pilot trial. Phytomedicine 1996;97;3:315-8. 43. Hancke J, Burgos R, Caceres D, Wikman G. A double-blind study with a new monodrug Kan Jang: decrease of symptoms and improvement in the recovery from common colds. Phytotherapy Res 1995;9:559-62. 44. Kulichenko LL, Kireyeva LV, Malyshkina EN, Wikman G. A Randomized, Controlled Study of Kan Jang versus Amantadine in the Treatment of Influenza in Volgograd. J Herb Pharmacother 2003;3:77-92. 45. Gabrielian ES, Shukarian AK, Goukasova GI, et al. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 2002;9:589-97. 46. Coon JT, Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta Med 2004;70:293-8. 47. Caceres DD, Hancke JL, Burgos RA, Wikman GK. Prevention of common colds with Andrographis Paniculata dried extract: a pilot, double-blind trial. Phytomedicine 1997;4:101-4. 48. Melchoir J, Spasov AA, Ostrovskij OV, et al. Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan Jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 2000;7:341-50. 49. Poolsup N, Suthisisang C, Prathanturarug S, et al. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther 2004;29:37-45. 50. Spasov AA, Ostrovskij OV, Chernikov MV, Wikman G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother Res 2004;18:47-53. 51. Asano K, Takahashi T, Miyashita M, et al. Effect of Eleutherococcus senticosus extract on human physical working capacity. Planta Med 1986;175-7. 52. Eschbach LF, Webster MJ, Boyd JC, et al. The effect of siberian ginseng (Eleutherococcus senticosus) on substrate utilization and performance. Int J Sport Nutr Exerc Metab 2000;10:444-51. 53. Dusman K, Plowman SA, McCarthy K, et al. The effects of Endurox on the physiological responses to stair-stepping exercise. Med Sci Sports Exerc 1998;30 Suppl:S323. 54. Cheuvroni SN, Moffatt RF, Biggerstaff KD, et al. Effects of Endurox on various metabolic responses to exercise. Med Sci Sports Exerc 1998;30 Suppl:S32. 55. Smerzer KD, Gretebeck PJ. Effect of radix Acanthopanax senticosus on submaximal running peformance. Med Sci Sports Excerc 1998;30 Suppl:S278. 56. Amaryan G, Astvatsatryan V, Gabrielyan E, et al. Double-blind, placebo-controlled, randomized, pilot clinical trial of ImmunoGuard--a standardized fixed combination of Andrographis paniculata Nees, with Eleutherococcus senticosus Maxim, Schizandra chinensis Bail. and Glycyrrhiza glabra L. extracts in patients with Familial Mediterranean Fever. Phytomedicine 2003;10:271-85. 57. Shang SY, Ma YS, Wang SS. [Effect of eleutherosides on ventricular late potential with coronary heart disease and myocarditis]. [Article in Chinese] Zhong Xi Yi Jie He Za Zhi 1991;11:280-1, 261. 58. Shi Z, Liu C, Li R. [Effect of a mixture of Acanthopanax senticosus and Elsholtzia splendens on serum-lipids in patients with hyperlipemia]. [Article in Chinese]. Zhong Xi Yi Jie He Za Zhi 1990r;10:155-6, 132. 59. Han L, Cai D. [Clinical and experimental study on treatment of acute cerebral infarction with Acanthopanax Injection]. [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998;18:472-4. 60. Davydov M, Krikorian AD. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: a closer look. J Ethnopharmacol 2000;72:345-93. 61. Harkey MR, Henderson GL, Gershwin ME, et al. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr 2001;73:1101-6. 62. Hacker B, Medon PJ. Cytotoxic effects of Eleutherococcus senticosus aqueous extracts in combination with N6-(delta 2-isopentenyl)-adenosine and 1-beta-D-arabinofuranosylcytosine against L1210 leukemia cells. J Pharm Sci 1984;73:270-2. 63. Szolomicki S, Samochowiec L, Wojcicki J, Drozdzik M. The influence of active components of Eleutherococcus senticosus on cellular defense and physical fitness in man. Phytother Res 2000;14:30-5. 64. Dasgupta A, Wu S, Actor J, et al. Effect of Asian and Siberian ginseng on serum digoxin measurement by five digoxin immunoassays. Significant variation in digoxin-like immunoreactivity among commercial ginsengs. Am J Clin Pathol 2003;119:298-303. 65. Medon PJ, Ferguson PW, Watson CF. Effects of Eleutherococcus senticosus extracts on hexobarbital metabolism in vivo and in vitro. J Ethnopharmacol 1984;10:235-41. 66. Glatthaar-Saalmuller B, Sacher F, Esperester A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antiviral Res 2001;50:223-8. 67. Shen ML, Zhai SK, Chen HL, Immunomopharmacological effects of polysaccharides from Acanthopanax senticosus on experimental animals. Int J Immunopharmacol 1991;13:549-54. 68. Mills S, Bone K. Principles and Practice of Phytotherapy. London: Churchill Livingstone, 2000. | |
Silver Birch
Scientific name:Betula pendula, Betula alba. Botanical family: Betulaceae. Parts used: The leaves and bark. Traditional use. Birch is used as a diuretic, for rheumatic ailments, for cystitis, for arthritis, rheumatism and as an alterative for aiding eliminatory processes. Safety. There are no safety concerns when used within the recommended dosages.1 Pregnancy: Consult with a medical herbalist. Breastfeeding: Consult with a medical herbalist. Constituents. Flavonoids; mainly hyperoside, luteolin and quercetin. Caffeic acid derivatives, including chlorogenic acid. Monoterpene glucosides; the betula albosides and roseoside. Saponins; betula triterpenes. Volatile oil containing betulin, caryophyllenes and methyl salicylate. Betulenols and their acetates. Anthocyanins. Resin; Tannins. Scientific evidence. No clinical research has been done. Mechanism of action. A study offers a rational basis for the use of Betula pendula leaf extract for the treatment of immune disorders, like rheumatoid arthritis, by diminishing proliferating inflammatory lymphocytes.5 The aquaretic and possibly saluretic (loss of salt through the kidneys) effects are due to the flavonoid constituents of the birch leaf. Aquaretics increase urine volume (water loss) but not electrolyte excretion.2 The high vitamin C content of the leaf can enhance the effect.3 An extract of betulinic acid may hold promise as an anticancer agent. Some laboratory and animal studies of betulinic acid have reported antitumor activity. Additional studies are under way to find out whether it has a role in treating several forms of cancer, including melanoma and certain brain cancers. Clinical trials are needed to determine what effect, if any, betulinic acid may have in treating cancer in humans.6,7,8,9,10,11,12 Adverse reactions. None reported. Interactions with herbs and supplements: None known. Interactions with drugs: None known. Interactions with foods: None known. Interactions with laboratory tests: None known. Dosage. Recommended dose: 3-10mls per day 1:5 tincture 30% alcohol. Infusion: range from 2-3tsps. per day. The recommended dose of Dr Clare’s Joint Support Blend provides 3mls per day of 1:3 Tincture in 15mls daily, at a dose of 5mls three times a day. This is equivalent to 375-750mgs per day. Dr Clare’s Joint Cleansing Tea provides ½ tsp. per day of peppermint. Dosage. Dr Clare’s Blends: 700mgs per day. Oral: The typical dose of birch leaf is several times daily as a tea, which is prepared by steeping 2-3 grams of finely cut dried leaf in approximately 150 mL boiling water for 10-15 minutes and straining.4,3 The tea should be taken with plenty of water.3 References. 1. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 2. Robbers JE, Tyler VE. Tyler's Herbs of Choice: The Therapeutic Use of Phytomedicinals. New York, NY: The Haworth Herbal Press, 1999. 3. Wichtl MW. Herbal Drugs and Phytopharmaceuticals. Ed. N.M. Bisset. Stuttgart: Medpharm GmbH Scientific Publishers, 1994. 4. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 5. Gründemann C1, Gruber CW, Hertrampf A, Zehl M, Kopp B, Huber R. J Ethnopharmacol. 2011 Jul 14;136(3):444-51. An aqueous birch leaf extract of Betula pendula inhibits the growth and cell division of inflammatory lymphocytes. 6. Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816-2823. 7. Eiznhamer DA, Xu ZQ. Betulinic acid: a promising anticancer candidate. IDrugs. 2004;7:359-373. 8. Jung GR, Kim KJ, Choi CH, et al. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol. 2007;101:277-285 9. Pisha E, Chai H, Lee IS, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046-1051. 10. Sawada N, Kataoka K, Kondo K, et al. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br J Cancer. 2004;90:1672-1678. 11. Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM. Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur J Cancer. 1997;33:2007-2010. 12. Thurnher D, Turhani D, Pelzmann M, et al. Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck. 2003;25:732-740. | |
Skullcap
American Skullcap, Blue Pimpernel, Blue Skullcap, Escutelaria, Helmet Flower, Scutellaria. Scientific Name: Scutellaria lateriflora. Family: Lamiaceae/Labiatae.
People Use This For: Skullcap is used for insomnia, anxiety, nervous tension, allergies, dermatitis, inflammation, and spasms.
Safety: There is insufficient scientific information available about the use of skullcap.
Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to RATE Anxiety. Preliminary evidence suggests that healthy people who take a single dose of skullcap extract might feel more relaxed than tense. This effect appears to last for about 2 hours.41 But it is not known if taking skullcap is effective for anxiety disorders or if extended use is beneficial. More scientific evidence is needed to rate skullcap for this use.
Mechanism of Action: The applicable parts of skullcap are the above ground parts. The principle flavonoids of skullcap are baicalin and wogonin.42,41 Skullcap also contains lignins, resins, tannins, and volatile oils.42 It is thought that the flavonoid constituents of skullcap might act as GABA agonists and therefore have a sedating and anxiolytic effect.41
Constituents of skullcap seem to bind the serotonin receptor 5-HT7. But it's not known if these constituents have agonist or antagonist properties on the receptor.42 Skullcap's effects on the serotonin receptor may be involved in potential sedative properties.
Adverse Reactions: Orally, single doses of skullcap extract appear to be well-tolerated. A single skullcap extract dose of 100 mg does not seem to affect cognition or cause significant sedation. But a higher dose of 200 mg might cause more sedation and cognitive impairment.41 The effect of these typical doses in repeated doses is not known.
Interactions with Herbs & Supplements: Herbs and Supplements with Sedative Properties: Theoretically, concomitant use with herbs that have sedative properties might enhance therapeutic and adverse effects.
Interactions with Drugs: None known.
Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: None known. Dosage/Administration: Dr Clare’s Blends: 1 gm per day
Oral: A typical dose is 1-2 grams or as a tea three times daily.43 The extract is typically used 2-4 mL (1:1 in 25% alcohol) three times daily. The tincture is typically used 3-6 mL (1:5 in 45% alcohol).43
Specific References: SKULLCAP 41. Wolfson P, Hoffmann DL. An investigation into the efficacy of Scutellaria lateriflora in healthy volunteers. Altern Ther Health Med 2003;9:74-8. 42. Gafner S, Bergeron C, Batcha LL, et al. Inhibitor of [3H]-LSD binding to 5-HT7 receptors by flavonoids from Scutellaria lateriflora. J Nat Prod 2003;66:535-7. 43. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996.
| |
Slippery ElmSlippery Elm | |
T |
|---|
Thyme
Common Thyme. Scientific Name: Thymus vulgaris; Thymus zygis. Family: Lamiaceae/Labiatae. People Use This For: Orally, thyme is used for bronchitis, whooping cough, sore throat. Topically, thyme is used for laryngitis, tonsillitis, stomatitis, and halitosis. Safety: Possibly Safe when used orally.145,146 Thyme has been used safely for 10 days.146 Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: INSUFFICIENT RELIABLE EVIDENCE to COMMENT Bronchitis. Clinical research suggests thyme, in combination with cowslip (Bronchipret), relieves symptoms of bronchitis such as coughing, fever, and increased production of sputum.146 Mechanism of Action: The applicable parts of thyme are the leaf, flower, and oil. Thyme contains the essential oils and several other constituents It also contains flavonoids, polyphenolic acids and other constituents.147,148 Thymol, the primary constituent of thyme is rapidly absorbed in the upper gastrointestinal tract. Preliminary research suggests that thyme has antimicrobial activity and modest antibacterial effects.149,150 It also seems to have antiviral activity.151 Other preliminary research suggests thyme has activity against fungi such as Candida albicans and other Candida species. Thymol is active against fungal microorganisms that cause fungal nail infections.152 Thyme and its constituents may have antioxidant effects.149,153,154 The antioxidant effect of thyme may increase the production of nitric oxide and improve atherosclerosis and endothelial dysfunction.155 Research suggests thyme has antispasmodic, antiplatelet, anti-inflammatory, and antiallergic activity.156,157,158,159 Additionally, thyme might improve wound healing.160 Adverse Reactions: Orally, thyme is generally well tolerated. Gastrointestinal adverse effects occur occasionally.146 Allergic reactions are uncommon.148 Interactions with Herbs & Supplements: None reported Interactions with Drugs: None reported Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None reported Dosage/Administration: Dr Clare’s Blends: 1 gm per day (3mls 1:3 Thyme tincture/day) Oral: For the treatment of bronchitis, a combination of thyme 160 mg and cowslip 54 mg (Bronchipret) has been used three times daily.146 Specific References: THYME 145. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol 1998;134:1349-46. 146. Ernst E, Marz R, Sieder C. A controlled multi-centre study of herbal versus synthetic secretolytic drugs for acute bronchitis. Phytomedicine 1997;4:287-93. 147. Kitajima J, Ishikawa T, Urabe A, Satoh M. Monoterpenoids and their glycosides from the leaf of thyme. Phytochemistry 2004;59:3279-87. 148. Spiewak R, Skorska C, Dutkiewicz J. Occupational airborne contact dermatitis caused by thyme dust. Contact Dermatitis 2001;38:235-9. 149. Proestos C, Chorianopoulos N, Nychas GJ, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem 2005;47:1190-5. 150. Hersch-Martinez P, Leanos-Miranda BE, Solorzano-Santos F. Antibacterial effects of commercial essential oils over locally prevalent pathogenic strains in Mexico. Fitoterapia 2005;76:453-7. 151. Kohlert C, Schindler G, Marz RW, et al. Systemic availability and pharmacokinetics of thymol in humans. J Clin Pharmacol 2002;36:731-7. 152. Ramsewak RS, Nair MG, Stommel M, Selanders L. In vitro antagonistic activity of monoterpenes and their mixtures against 'toe nail fungus' pathogens. Phytother Res 2003;17:376-9. 153. Aydin S, Basaran AA, Basaran N. Modulating effects of thyme and its major ingredients on oxidative DNA damage in human lymphocytes. J Agric Food Chem 2005;47:1299-305. 154. Agbor GA, Oben JE, Ngogang JY, et al. Antioxidant capacity of some herbs/spices from cameroon: a comparative study of two methods. J Agric Food Chem 2005;47:6819-18. 155. Grande S, Bogani P, de Saizieu A, et al. Vasomodulating potential of mediterranean wild plant extracts. J Agric Food Chem 2004;46:5021-6. 156. Meister A, Bernhardt G, Christoffel V, Buschauer A. Antispasmodic activity of Thymus vulgaris extract on the isolated guinea-pig trachea: discrimination between drug and ethanol effects. Planta Med 1999;59:512-6. 157. Okazaki K, Kawazoe K, Takaishi Y. Human platelet aggregation inhibitors from thyme (Thymus vulgaris L.). Phytother Res 2002;16:398-9. 158. Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A.1 murine macrophages. J Pharm Pharmacol 2004;50:257-57. 159. Watanabe J, Shinmoto H, Tsushida T. Coumarin and flavone derivatives from estragon and thyme as inhibitors of chemical mediator release from RBL-2H3 Cells. Biosci Biotechnol Biochem 2005;69:1-6. 160. Dursun N, Liman N, Ozyazgan I, et al. Role of thymus oil in burn wound healing. J Burn Care Rehabil. 2003;18:395-9.
| |
Turkey Rhubarb
Scientific Name: People Use This For: Safety: Effectiveness: Mechanism of Action: Adverse Reactions: Interactions with Herbs & Supplements: Interactions with Drugs: Interactions with Foods: Interactions with Lab Tests: Interactions with Diseases or Conditions: Dosage/Administration: Specific References: Turkish Rhubarb | |
U |
|---|
Uva-ursi
Bearberry, Beargrape, Arctostaphylos uva-ursi. Family: Ericacea. People Use This For: Orally, uva ursi is used for urinary tract infections, inflammatory conditions of the urinary tract, cystitis, urethritis, diuresis, constipation, stones in the urinary tract, painful urination, acidic urine, kidney infection, benign prostatic hyperplasia.
Safety: No concerns regarding safety when used orally and appropriately, short-term. Preliminary clinical research suggests that uva ursi is likely to be safe when used short-term.13,14 Possibly unsafe when used orally long-term because of the hydroquinone constituent of uva ursi. Pregnancy and Lactation: Refer to a Medical Herbalist Effectiveness: Not enough scientific information gather to offer a comment. Urinary tract infections (UTIs). Preliminary evidence suggests that taking a combination product containing both uva ursi and dandelion orally seems to significantly reduce the recurrence rate of UTIs in women.14 However, since it isn't clear if this kind of extended use is safe, advise patients not to use uva ursi for long-term prevention of UTIs. Mechanism of Action: Arbutin hydrolyses to hydroquinone which is an effective urinary antiseptic.R9 It is also cytotoxic and mutagenic and so excessive doses should be avoided.R10 Arbutin is absorbed from the gastrointestinal tract unchanged and is hydrolyzed to hydroquinone in alkaline urine. There it can exert antiseptic and astringent effects.15,16,17 For this reason, urinary acidifying agents may decrease the effectiveness of uva ursi, and urinary alkalinizing agents may increase its effectiveness.18 Hydroquinone, contained in uva ursi, is a tyrosine kinase inhibitor which inhibits melanin production. Melanin is present in various ocular layers . Changes in melanin metabolism may result in retinal thinning19 (This was one case following 3 years use). Hydroquinone is cytotoxic in lab studies.15 In rats, uva ursi has anti-inflammatory activity against experimentally-induced inflammation.15 Adverse Reactions: Orally, uva ursi may cause nausea, vomiting, gastrointestinal discomfort, and a greenish-brown discoloration of the urine.20,15 Interactions with Herbs & Supplements: None known. Interactions with Drugs: Lithium: Due to diuretic properties which theoretically may affect the dose of lithium required. Refer to a Medical Herbalist. Interactions with Foods: None known. Interactions with Lab Tests: Colorimetric Urine Tests: Theoretically, uva ursi can interfere with colorimetric urine tests and can turn urine greenish-brown.15 Interactions with Diseases or Conditions: Retinal Thinning: Theoretically, uva ursi might worsen retinal thinning in patients with this condition. Long-term use of uva ursi should be avoided in these patients.19 Dosage/Administration: Oral: The typical dose of dried herb is 1.5-4 grams daily.15 It's also commonly used as a tea. The tea is prepared by steeping 3 grams of the dried leaf in 150 mL cold water for 12-24 hours and then straining. One cup of tea is usually taken up to 4 times daily.20,17,21 Uva ursi leaf teas should be prepared with cold water to minimize the tannin content.21 The hydroquinone derivative, calculated as water-free arbutin, is commonly dosed as 100-210 mg up to 4 times daily.20,17,21 The fluid extract (1:1 in 25% alcohol) is given 1.5-4 mL three times daily.15 Medical consultation is needed for urinary tract symptoms persisting longer than 48 hours.15 Uva ursi should not be used longer than one week without monitoring due to its potential risks.17 Limit its use to less than five times per year.17
Spedific References: UVA URSI 13. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 14. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res 1993;53:441-3. 15. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 16. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 17. Schulz V, Hansel R, Tyler VE. Rational Phytotherapy: A Physician's Guide to Herbal Medicine. Terry C. Telger, transl. 3rd ed. Berlin, GER: Springer, 1998. 18. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998. 19. Wang L, Del Priore LV. Bull's-eye maculopathy secondary to herbal toxicity from uva ursi. Am J Ophthalmol 2004;137:1135-7. 20. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 21. Foster S, Tyler VE. Tyler's Honest Herbal, 4th ed., Binghamton, NY: Haworth Herbal Press, 1999. | |
V |
|---|
Valerian
All-Heal, Common Valerian, Fragrant Valerian, Garden Heliotrope,
Scientific Name:Valeriana officinalis; Valeriana edulis; Valeriana angustifolia; Valeriana jatamansii, synonym Valeriana wallichii; Valeriana sitchensis. Family: Valerianaceae. People Use This For: Valerian is used for insomnia, and for anxiety-associated restlessness and sleeping disorders. It has also been used for mood disorders such as depression, and chronic fatigue syndrome (CFS). It is also used orally for muscle and joint pain, and conditions associated with anxiety and psychological stress including nervous asthma, excitability, headaches, migraine, and stomach upset. Valerian is also used for menstrual cramps and symptoms associated with menopause, including hot flashes and anxiety. In manufacturing, the extracts and essential oil are used as flavoring in foods and beverages.
Safety: No concerns regarding safety when used in amounts commonly found in foods. Valerian has Generally Recognized as Safe (GRAS) status in the US.60 No concerns regarding safety when used orally and appropriately, short-term. Clinical studies have reported safe use of valerian for medicinal purposes in over 12,000 patients in trials lasting up to 28 days.61,62,63,64,65,66 The safety of long-term use is unknown. Children: No concerns regarding safety when used orally and appropriately, short-term. Valerian has been safely used in children in studies lasting 4-8 weeks.74,80 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: POSSIBLY EFFECTIVE Insomnia. Most research shows that taking valerian orally reduces the time to sleep onset (sleep latency), and improves subjective sleep quality. The greatest benefit is usually seen in patients using 400-900 mg valerian extract up to 2 hours before bedtime.67,68,69,70 Valerian does not relieve insomnia as fast as benzodiazepines.65 Continuous nightly use for several days to four weeks might be needed for significant effect.68,71 Valerian is often used in combination with other sedative herbs. Taking a combination product containing valerian extract 187 mg plus hops extract 41.9 mg per tablet, two tablets at bedtime, seems to modestly improve subjective sleep measures including subjective sleep latency compared to placebo after 28 days of treatment; however it was not significantly better than placebo after only 14 days of treatment.66 A combination of valerian with lemon balm might also improve the quality and quantity of sleep in healthy people.72 Valerian also seems to improve the sleep quality of insomniacs who have recently withdrawn from benzodiazepines. After tapering the benzodiazepine over two weeks, 300 mg valerian extract in three divided daily doses might subjectively improve sleep quality.73 There is also preliminary clinical research that suggests valerian improves sleep in intellectually impaired children.74 Not all evidence is positive. Some evidence suggests that valerian does not significantly improve insomnia compared to placebo.79 INSUFFICIENT RELIABLE EVIDENCE to RATE Anxiety. There is contradictory evidence about the effectiveness of valerian for anxiety. Some evidence shows that taking valerian orally reduces self-reported stress in social anxiety.75,76 However, another preliminary study found no significant difference in anxiety scores in patients with generalized anxiety disorder compared to placebo.77,78 Additional preliminary evidence suggests that valerian does not significantly decrease anxiety compared to placebo.79 Restless Sleep. Preliminary (980 Participants open trial :definite benefit) evidence suggests that a specific combination product providing valerian root extract 160 mg and lemon balm leaf extract 80 mg (Euvegal forte, Dr. Willmar Schwabe Pharmaceuticals) 1-2 tablets once or twice daily might decrease symptoms in children under age 12 years who have pathological restlessness or dyssomnia.80 More evidence is needed to rate valerian for these uses. Mechanism of Action: The applicable part of valerian is the root and rhizome. Valerian is thought to have sedative-hypnotic, anxiolytic, antidepressant, anticonvulsant, and antispasmodic effects.62,63,81 Valerian might also have hypotensive properties.75 The pharmacological effects of valerian have primarily been attributed to valepotriates.62,82,83 Other potentially active constituents include flavonoids.83 Because valerian extracts without some of these constituents can still have similar effects, it is likely that multiple constituents are responsible for its pharmacological effects. Valepotriate constituents have sedative-hypnotic and spasmolytic effects. Valepotriates have also been shown to decrease benzodiazepine withdrawal in an animal model and to bind dopamine receptors.62,82 The valepotriates might act as prodrugs.82 The sesquiterpenes have been shown to cause sedation in animals.83 Valerenic acid and other constituents of valerian may increase GABA (neurotransmitter) concentrations and decrease central nervous system activity.82,84 Valerian may stimulate the release and reuptake of GABA84 Valerian might also affect sleep regulation with activity on adenosine and 5-hydroxytryptamine-1.84 The valerian flavonoid constituents also seem to have a role in the sedative-hypnotic effects of valerian.83 Adverse Reactions: A few patients can find that valerian is a stimulant and they should avoid its use. (This is generally at higher doses (DC).) In some individuals, valerian can aggravate a sensation of drowsiness or tiredness, particularly at higher doses, but this is usually a case of an increased awareness of the body’s needs than a negative depressant effect.R1 pp.587 Valerian can cause headache, excitability, uneasiness, palpitations, and insomnia.62 Occasionally, valerian may cause gastric discomfort, dry mouth, vivid dreams,64 and morning drowsiness.61 Taking valerian extracts in doses up to 1800 mg does not appear to significantly affect mood or psychomotor performance.85,86 Valerian does not usually have a negative impact on reaction time, alertness, and concentration the morning after intake. Impairment that does occur is dose-dependent and seems to peak within the first few hours after an oral valerian dose.61 Interactions with Herbs & Supplements: Herbs and Supplements with Sedative Properties: Use of valerian with other herbs and supplements with sedative properties might enhance therapeutic and adverse effects.82 Interactions with Drugs: Alchol (Ethanol): Alcohol may be used as a CNS depressant which reduces performance of motor skills. Do not add the sedative effects of Valerian.87 Anxiolytics: Patients may require less medication for anxiety if they use herbal remedies with all of their attendant side effects but do not combine them within 4-5 hours of each other. Interactions with Foods: None known. Interactions with Lab Tests: None known. Interactions with Diseases or Conditions: None Known.
Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture. ORAL: For insomnia, most studies have used 400-900 mg valerian extract up to 2 hours before bedtime for as long as 28 days.61,62,65,73,69,70,85 Other studies have used valerian extract 300-450 mg given in three divided doses.62,73Valerian should be given 30 minutes to 2 hours before bedtime.87 Specific References: VALERIAN 60. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 61. Kuhlmann J, Berger W, Podzuweit H, Schmidt U. The influence of valerian treatment on "reaction time, alertness and concentration" in volunteers. Pharmacopsychiatry 1999;32:235-41. 62. Klepser TB, Klepser ME. Unsafe and potentially safe herbal therapies. Am J Health Syst Pharm 1999;56:125-38. 63. Plushner SL. Valerian: Valerian officinalis. Am J Health Syst Pharm 2000;57:328,333,335. 64. Wheatley D. Stress-induced insomnia treated with kava and valerian: singly and in combination. Hum Psychopharmacol 2001;16:353-6. 65. Dorn M. [Efficacy and tolerability of Baldrian versus oxazepam in non-organic and non-psychiatric insomniacs: a randomized, double-blind, clinical, comparative study]. [Article in German]. Forsch Komplementarmed Klass Naturheilkd 2000;7:79-84. 66. Morin CM, Koetter U, Bastien C, et al. Valerian-hops combination and diphenhydramine for treating insomnia: a randomized placebo-controlled clinical trial. Sleep 2005;28:1465-71. 67. Leathwood PD, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav 1982;17:65-71. 68. Donath F, Quispe S, Diefenbach K, et al. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsych 2000;33:47-53. 69. Bent S, Patterson M, Garvin D. Valerian for sleep: a systematic review and meta-analysis. Alternative Therapies 2001;7:S4. 70. Leathwood PD, Chauffard F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Med 1985;2:144-8. 71. Stevinson C, Ernst E. Valerian for insomnia: a systematic review of randomized clinical trials. Sleep Med 2000;1:91-9. 72. Cerny A, Shmid K. Tolerability and efficacy of valerian/lemon balm in healthy volunteers (a double blind, placebo-controlled, multicentre study). Fitoterapia 1999;70:221-8. 73. Poyares DR, Guilleminault C, Ohayon MM, Tufik S. Can valerian improve the sleep of insomniacs after benzodiazepine withdrawal? Prog Neuropsychopharmacol Biol Psychiatry 2002;26:539-45. 74. Francis AJ, Dempster RJ. Effect of valerian, Valeriana edulis, on sleep difficulties in children with intellectual deficits: randomised trial. Phytomedicine 2002;9:273-9. 75. Cropley M, Cave Z, Ellis J, Middleton RW. Effect of kava and valerian on human physiological and psychological responses to mental stress assessed under laboratory conditions. Phytother Res 2002;16:23-7. 76. Kohnen R, Oswald WD. The effects of valerian, propranolol, and their combination on activation, performance and mood of healthy volunteers under social stress conditions. Pharmacopsychiatry 1988;21:447-8. 77. Andreatini R, Sartori VA, Seabra ML, Leite JR. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res 2002;16:650-4. 78. Miyasaka LS, Atallah AN, Soares BG. Valerian for anxiety disorders. Cochrane Database Syst Rev 2006;(4):CD004515. 79. Jacobs BP, Bent S, Tice JA, et al. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine (Baltimore) 2005;84:197-207. 80. Muller SF, Klement S. A combination of valerian and lemon balm is effective in the treatment of restlessness and dyssomnia in children. Phytomedicine 2006;13:383-7. 81. Eadie MJ. Could valerian have been the first anticonvulsant? Epilepsia 2004;45:1338-43. 82. Houghton PJ. The scientific basis for the reputed activity of Valerian. J Pharm Pharmacol 1999;51:505-12. 83. Fernandez S, Wasowski C, Paladini AC, Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol Biochem Behav 2004;77:399-404 84. Yuan CS, Mehendale S, Xiao Y, et al. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth Analg 2004;98:353-8. 85. Glass JR, Sproule BA, Herrmann N, et al. Acute pharmacological effects of temazepam, diphenhydramine, and valerian in healthy elderly subjects. J Clin Psychopharmacol 2003;23:260-8. 86. Gutierrez S, Ang-Lee MK, Walker DJ, Zacny JP. Assessing subjective and psychomotor effects of the herbal medication valerian in healthy volunteers. Pharmacol Biochem Behav 2004;78:57-64. 87. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
| |
VerbenaAlso Known As: Common Verbena, Common Vervain, Herb of Grace, Herb of the Cross,
Scientific Name: Verbena officinalis. Family: Verbenaceae.
People Use This For: Verbena is used for sore throats. Verbena is also used orally for depression, melancholia, and convalescence after fevers. It is also used for pain, spasms, exhaustion, nervous conditions, digestive disorders, liver and gallbladder diseases. Other uses include menopausal complaints, irregular menstruation, increasing lactation.
Safety: No concerns regarding safety when used orally in amounts commonly found in foods. Verbena has Generally Recognized As Safe status (GRAS) for use in foods in the US.88
Pregnancy and Lactation: Refer to a Medical Herbalist.
Effectiveness: POSSIBLY EFFECTIVE Sinusitis. Taking verbena orally in combination with gentian root, elderflower, cowslip flower, and sorrel (SinuComp, Sinupret) is effective for treating acute or chronic sinusitis. Clinical studies have used Sinupret.89,90
Mechanism of Action: The applicable parts of verbena are the above ground parts. Verbena contains verbascoside (acetoside), verbenalin, beta-sitosterol, ursolic acid, oleanolic acid, citral, and other constituents.91,92 Preliminary research suggests verbena might have anti-inflammatory and weak antimicrobial activity.93,91 Other preliminary research suggests constituent verbascoside has analgesic, sedative effects, antioxidant, and hepatoprotective effects.94,95,96
Adverse Reactions: Verbena appears to have low toxicity.R2 pp.442
Interactions with Herbs & Supplements: None known.
Interactions with Drugs: None known. Interactions with Foods: None known.
Interactions with Lab Tests: None known.
Interactions with Diseases or Conditions: None known.
Dosage/Administration: Dr Clare’s Blends: Dose 455mgs per day. 1.5mls 1:3 Tincture.
Dried Herb 2-4gms per day.R2 pp.442
Specific References: VERBENA 88. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 89. Neubauer N, Marz RW. Placebo-controlled, randomized, double-blind, clincal trial with Sinupret sugar coated tablets on the basis of a therapy with antibiotics and decongestant nasal drops in acute sinusitis. Phytomedicine 1994;1:177-81. 90. Marz RW, Ismail C, Popp MA. Action profile and efficacy of a herbal combination preparation for the treatment of sinusitis. Wien Med Wochenschr 1999;149:202-8. 91. Deepak M, Handa SS. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother Res 2000;14:463-5. 92. Dudai N, Weinstein Y, Krup M, et al. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med 2005;71:484-8 93. Hernandez NE, Tereschuk ML, Abdala LR. Antimicrobial activity of flavonoids in medicinal plants from Tafi del Valle (Tucuman, Argentina). J Ethnopharmacol 2000;73:317-22. 94. Nakamura T, Okuyama E, Tsukada A, et al. Acteoside as the analgesic principle of Cedron (Lippia triphylla), a Peruvian medicinal plant. Chem Pharm Bull 1997;45:499-504. 95. Chiou WF, Lin LC, Chen CF. Acteoside protects endothelial cells against free radical-induced oxidative stress. J Pharm Pharmacol 2004;56:743-8. 96. Lee KJ, Woo ER, Choi CY, et al. Protective effect of acteoside on carbon tetrachloride- induced hepatotoxicity. Life Sci 2004;74:1051-64. | |
W |
|---|
White Horehound
Scientific Name: People Use This For: Safety: Effectiveness: Mechanism of Action: Adverse Reactions: Interactions with Herbs & Supplements: Interactions with Drugs: Interactions with Foods: Interactions with Lab Tests: Interactions with Diseases or Conditions: Dosage/Administration: Specific References: WhiteHorehound
| |
WHITE WILLOW
Basket Willow, Bay Willow, Black Willow, Black Willowbark, Black Willow Extract, European Willow Bark. Scientific Name: Salix alba; other Salix species. Family: Salicaceae. People Use This For: Willow bark is used for headache, pain, muscle pain, osteoarthritis, painful periods, gouty arthritis, ankylosing spondylitis, rheumatoid arthritis, and gout. It is also used for fever, common cold, and influenza. Safety: No concerns regarding safety when used orally and appropriately, short-term.19,20,21,22,23 Willow bark has been used safely for up to 13 weeks in one study.23
Children: Possibly Unsafe.24 Although Reye's syndrome has not been reported, the salicin constituent in willow bark is similar to aspirin and might pose the same risk. Pregnancy and Breastfeeding: Consult a Medical Herbalist. Effectiveness: POSSIBLY EFFECTIVE Back pain. There's some evidence that a willow bark extract providing 120-240mg of the salicin constituent daily can reduce low back pain in some patients. The higher concentration of 240mg salicin is more effective than 120mg of salicin. It can take up to 1 week for significant relief.19 Some research suggests salicin 240mg daily is as effective as rofecoxib (Vioxx) for low back pain.22 INSUFFICIENT RELIABLE EVIDENCE to RATE Osteoarthritis. Clinical research on willow bark extract for osteoarthritis is conflicting. Some research suggests it has a moderate analgesic effect on osteoarthritis, while other research shows it is similar to placebo and inferior to diclofenac (Voltaren, Cataflam).20,21 Rheumatoid arthritis (RA). Preliminary clinical research suggests that willow bark extract is not effective for rheumatoid arthritis.21 More evidence is needed to rate willow bark for these uses. Mechanism of Action: Willow bark is the bark of salix tree species such as the white willow. Willow bark constituents include flavonoids, tannins, and salicylates. The active constituent of willow bark is thought to be salicin. Salicin is metabolized to salicyl alcohol and then to salicylic acid. From there, metabolism is the same as aspirin.25 An ethanolic extract of willow bark seems to inhibit cyclooxygenase (COX)-2 mediated prostaglandin release, but it doesn't seem to directly affect COX-1 or COX-2 activity. Constituents of willow bark other than salicin may have lipoxygenase-inhibiting and antioxidant effects that could contribute to its analgesic effect.19,26 They also might prevent prostaglandin and cytokine release.26 Some research suggests that extended treatment with willow bark may be necessary for a therapeutic effect.27,28 Preliminary research suggests that willow bark extracts have analgesic, anti-inflammatory, and antipyretic effects.26 Willow bark inhibits platelet aggregation, but to a lesser degree than aspirin.29 Bioavailability of willow bark may be lower due to manufacturing processes. A dose of 240mg of salicin is equivalent to 87mg of aspirin.25 Adverse Reactions: Willow bark extract can cause gastrointestinal adverse effects, but these appear to be less frequent than those caused by NSAIDs.20,21 Willow bark may cause itching and rash, as well as serious allergic reactions, including anaphylaxis, in people who are allergic to aspirin.30,20,21 Salicylates can inhibit prostaglandins, which can reduce renal blood flow.31 Salicin can cause renal papillary necrosis.32 The risk for toxicity is greater with high acute doses or chronic use.31 Interactions with Herbs & Supplements: Anticoagulant/Antiplatelet Herbs and Supplements: Concomitant use of herbs that have antiplatelet/anticoagulant effects could theoretically increase the risk of bleeding in some people.29 These herbs include clove, danshen, garlic, ginger, ginkgo, ginseng, meadowsweet, red clover, and others. Salicylate-Containing Herbs: Theoretically, concomitant use may potentiate salicylate effects and adverse effects.25 Salicylate-containing herbs include aspen bark, black haw, poplar, and meadowsweet. Interactions with Drugs: Warfarin: Absolute contraindication. Aspirin: Willow bark contains salicin, a plant salicylate. Theoretically, willow bark might have an additive effect with other salicylate-containing drugs such as aspirin.25 Choline Magnesium Trisalicylate (Trilisate): Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate. Occurrence = Probable. Willow bark contains salicin, a plant salicylate. Theoretically, willow bark might have an additive effect with other salicylate-containing drugs such as choline magnesium trisalicylate.25 Salsalate (Disalcid): Interaction Rating = Moderate Be cautious with this combination. Severity = Moderate. Occurrence = Probable. Willow bark contains salicin, a plant salicylate. Theoretically, willow bark might have an additive effect with other salicylate-containing drugs such as salsalate.25 Interactions with Foods: None known. Interactions with Lab Tests: Creatinine: Theoretically, salicin could cause a rise in serum creatinine without affecting renal function. Salicylates can cause a rise in serum creatine without a change in glomerular filtration rate (GFR). This is thought to be due to changes in plasma protein binding caused by salicylates or competitive inhibition of tubular secretion of creatinine by salicylates.33 Interactions with Diseases or Conditions: Kidney Dysfunction: Theoretically, salicin might reduce renal blood flow.31 Chronic use of high doses might contribute to renal failure in predisposed people.32 Avoid use in people with compromised renal function. Salicylate Precautions: Avoid or use cautiously in individuals with aspirin hypersensitivity, asthma, active peptic ulcer disease, diabetes, gout, hemophilia, hypoprothrombinemia, kidney or liver disease. Willow bark may cause serious allergic reactions, including anaphylaxis, in people who are allergic to aspirin.30 Surgery: discontinue willow bark at least 2 weeks before elective surgical procedures. Dosage/Administration: Dr Clare’s Blend: 700mgs/day Oral: For back pain, willow bark extract providing 120-240mg salicin has been used. The higher 240mg dose might be more effective.19,22 Specific References: WHITE WILLOW 19. Chrubasik S, Eisenberg E, Balan E, et al. Treatment of low back pain exacerbations with willow bark extract: a randomized double-blind study. Am J Med 2000;109:9-14. 20. Schmid B, Ludtke R, Selbmann HK, et al. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: randomized placebo-controlled, double blind clinical trial. Phytother Res 2001;15:344-50. 21. Biegert C, Wagner I, Ludtke R, et al. Efficacy and safety of willow bark extract in the treatment of osteoarthritis and rheumatoid arthritis: results of 2 randomized double-blind controlled trials. J Rheumatol 2004;31:2121-30. 22. Chrubasik S, Kunzel O, Model A, et al. Treatment of low back pain with a herbal or synthetic anti-rheumatic: a randomized controlled study. Willow bark extract for low back pain. Rheumatology (Oxford) 2001;40:1388-93. 23. Coffey CS, Steiner D, Baker BA, Allison DB. A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int J Obes Relat Metab Disord 2004;28:1411-9. 24. Food and Drug Administration, HHS. Labeling for oral and rectal over-the-counter drug products containing aspirin and nonaspirin salicylates; Reye's Syndrome warning. Final rule. Fed Regist 2003;18:18861-9. 25. Schmid B, Kotter I, Heide L. Pharmacokinetics of salicin after oral administration of a standardised willow bark extract. Eur J Clin Pharmacol. 2001;57:387-91. 26. Fiebich BL, Chrubasik S. Effects of an ethanolic salix extract on the release of selected inflammatory mediators in vitro. Phytomedicine 2004;11:135-8. 27. Wagner I, Greim C, Laufer S, et al. Influence of willow bark extract on cyclooxygenase activity and on tumor necrosis factor alpha or interleukin 1 beta release in vitro and ex vivo. Clin Pharmacol Ther 2003;73:272-14. 28. Fiebich BL, Appel K. Anti-inflammatory effects of willow bark extract. Clin Pharmacol Ther 2003;74:96. 29. Krivoy N, Pavlotzky E, Chrubasik S, et al. Effect of salicis cortex extract on human platelet aggregation. Planta Med 2001;17:209-13. 30. Boullata JI, McDonnell PJ, Oliva CD. Anaphylactic reaction to a dietary supplement containing willow bark. Ann Pharmacother 2003;37:832-5. 31. D'Agati V. Does aspirin cause acute or chronic renal failure in experimental animals and in humans? Am J Kidney Dis 1996;28:S24-9. 32. Schwarz A. Beethoven's renal disease based on his autopsy: a case of papillary necrosis. Am J Kidney Dis 1993;21:643-52. 33. Andreev E, Koopman M, Arisz L. A rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible? J Intern Med 1999;246:247-52. | |
Wild Oats
Avena, Oatstraw, Wild Oat Herb. Scientific Name: Avena sativa. Family: Poaceae/Gramineae. People Use This For: Oats are also used for acute or chronic anxiety, excitation and stress. Nervous exhaustion, depressive states, insomnia.R1 pp.234 menopause.R2 pp.317 Safety: No concerns regarding safety when used orally and appropriately.69,70,71,72 Oat bran has Generally Recognized as Safe (GRAS) status in the US.73 No concerns regarding safety when used topically and appropriately.74 Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: Insufficient evidence to comment on the effectiveness for stress. Mechanism of Action: The applicable parts of oats are the seeds and straw. There is no research done on the effects on nervous exhaustion or mood. Adverse Reactions: Orally, oats are usually very well tolerated. Interactions with Herbs & Supplements: None known. Interactions with Drugs: None known. Interactions with Foods: None known. Interactions with Lab Tests: See below for Coeliac Disease. Interactions with Diseases or Conditions: Celiac Disease: Oats and oat bran are generally excluded from gluten-free diets. However, oat products that are not contaminated with wheat, rye, or barley do not appear to cause adverse effects in nutrition, intestinal histology, or serology in adults with celiac disease in remission.75 Dosage/Administration: Oral: Traditionally milky oat pods are also used as a tincture and tea. Topical: No typical dosage. Dr Clare’s Comments: Although there is unlikely to be any problem with Wild Oat infusion or tincture for celiac patients I don’t prescribe it because patients feel anxious about it in the remedy. This is traditionally used as a very nourishing herb for stamina in the nervous system. Particularly good for exhaustion from chronic stress especially with a poor sleeping pattern. It is suitable for all ages. Specific References: OATS 69. FDA Talk Paper. FDA Allows Whole Oat Foods to make Claim on Reducing the Risk of Heart Disease. 1997. Available at: vm.cfsan.fda.gov/~lrd/tpoats.html. 70. American Dietetic Association Website. Available at: www.eatright.org/adap1097.html (Accessed 16 July 1999). 71. Food and Drug Administration. Food labeling: health claims: oats and coronary heart disease. Fed Regist 1996;61:296-313. 72. Foulke J. FDA Allows Whole Oat Foods To Make Health Claim on Reducing the Risk of Heart Disease. FDA Talk Paper. 1997. Available at: http://www.fda.gov/bbs/topics/ANSWERS/ANS00782.html. 73. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. 74. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 75. Storsrud S, Olsson M, Arvidsson Lenner R, et al. Adult coeliac patients do tolerate large amounts of oats. Eur J Clin Nutr 2003;57:163-9.
| |
Y |
|---|
Yarrow
Scientific name:Achillea millefolium, Botanical Family: Asteraceae/Compositae. Part used: The above ground parts. Traditional use. Legend has it that yarrow (Achillea millefolium) was named after Achilles, the Greek mythical hero who used it to stop the bleeding wounds of his soldiers. Some First American tribes used yarrow as a body-wash before battles because it was known to reduce bleeding from wounds. As a traditional medicine yarrow is used as a blood cleanser via the induction of sweating and promoting urine flow. Yarrow lowers fever, acts as an anti-inflammatory, it staunches bleeding from small blood vessels, and has antispasmodic and hypotensive qualities. It is used for feverish illnesses including, the common cold and influenza, chronic ‘runny’ nose, absence of or scanty menstrual periods, dysentery, diarrhea, loss of appetite, mild or crampy discomfort of the digestive tract, promotion of sweating and reduce high blood pressure. It is used for toning the venous system and reducing congestion of the lower limbs and pelvis by enhancing venous return. It is used throughout the cycle for heavy periods and during menses to reduce heavy bleeding. In combination with other herbs, yarrow is used for bloating, flatulence, mild gastrointestinal cramping, and nervous digestive complaints. In foods, the young leaves and flowers of yarrow are used in salads. Safety. No concerns regarding safety when used orally in amounts commonly found in foods. Yarrow has Generally Recognized As Safe (GRAS) status for use in foods in the US.1 No concerns regarding safety have been reported with medicinal use of yarrow.2,3 Pregnancy:avoid in pregnancy because its ability to relax the smooth muscle of the uterus may be associated with miscarriage. Breastfeeding: because very little information is available avoid excessive amounts. Unless advised by a medical herbalist for clinical reasons that outweigh the lack of information do not take more than 1 tsp per day. Constituents. Volatile oil; azulene, chamazulene,guaiizulene, alpha and beta pinene, borneol, bornyl acetate, camphor, caryophyllene, eugenol, farnesene, myrcene, sabinene, isoartemisia ketone, terpineol, thujone, alpha-bisabolol, neridol, spathulenol and others. Sesquiterpenes and sesquiterpene lactones; achillin, achillicin, hydroxachillen, balchanolide, leucodin, millifin, millifolide, longipine and achillifolin and their derivatives. Flavonoids; apigenin, luteolin, quercitin and their glucosides. Alkaloids and bases; betonicene, stachydrine, achiceine, moschatine, trigonelline and others. Miscellaneous; polyvynes, cyclitols, salicylic acid etc. Scientific evidence. There is evidence of effectiveness of yarrow for reducing high blood pressure in anaesthetised rats.[1] There are no clinical studies on the use of Yarrow for osteoarthritis. Mechanism of action. Yarrow has an evidence base for promoting sweat, lowering fever, lowering blood pressure, astringency, promotion of urine flow, urinary antiseptic, antispasmodic, and antiflatulent effects.4 Yarrow contains amino acids, fatty acids, ascorbic acid (vitamin C), caffeic acid, folic acid, salicylic acid, succinic acid, alkaloids, flavonoids including rutin, tannins, volatile oil, an unknown cyanogenetic compound, and sugars.4 The volatile oil contains chamazulene, other azulenes,5 and trace amounts of thujone.4,5 The volatile oil content, and especially the azulene content, varies considerably depending on the source.5 The alkaloid fraction has shown evidence of fever lowering and blood pressure lowering effects.4 A water extract shows some evidence of anti-inflammatory and diuretic activity. 4 The anti-inflammatory activity is partly mediated by inhibition of protein splitting enzymes involved in the inflammatory pathways (HNE and MMP-2 and -9).9 Anti-inflammatory and anti-allergy activities may be associated with the volatile oil chamazulene.6 Not all species contain azulene constituents.6 An alcohol extract shows moderate antibacterial effects. Animal studies showed protective anti-stomach ulcer effect which included protecting the stomach lining against indomethacin (An NSAID).8 Interactions with herbs and supplements. None reported. Because yarrow contains the essential oil thujone in very small amounts there is a possibility that taking other herbs containing this essential oil will have an additive effect leading to problems associated with toxicity of high levels of thujone. Thujone-containing herbs include oak moss, oriental arborvitae, sage, tansy, thuja (cedar), tree moss, and wormwood. Be careful to avoid higher than therapeutic doses. Consult a medical herbalist for advice regarding combining these herbs. The level of thujone in yarrow used by itself of not a concern. Interactions with drugs. Warfarin: there is a theoretical risk of interaction. Monitor weekly and do not exceed the therapeutic dose. Lithium. Precaution advised with any herb or drug with diuretic effect including yarrow, black tea and coffee. If you start of stop any substance with a diuretic action monitor your blood lithium level. Interactions with foods. None known. Interactions with lababoratory tests. None known. Dosage. Recommended dose: Tincture: 2-6mls per day 1:5 tincture 30% alcohol. Infusion: 1-3 tsps per day. Powder/capsule: 1-2gms per day in tablet form. Infusion of dried herb: 2-4 gms per day. Typically 1 tsp. three times daily as a herb tea. This can be increased to 1 tsp. every 2 hours for acute conditions such as influenza. Liquid extract: 1-3ml/day 1:1 in 25% alcohol. Dr Clare’s Joint Cleansing Tea provides ½ tsp. per day of peppermint. References. 1. FDA. Center for Food Safety and Applied Nutrition, Office of Premarket Approval, EAFUS: A food additive database. Available at: vm.cfsan.fda.gov/~dms/eafus.html. [Accessed 22/6/2014] 2. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S. Klein. Boston, MA: American Botanical Council, 1998. 3. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 4. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 5. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd ed. New York, NY: John Wiley & Sons, 1996. 6. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999 7. Blood pressure lowering, cardiovascular inhibitory and bronchodilatory actions of Achillea millefolium. Phytother Res. 2011 Apr;25(4):577-83. 8. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats Ana Maria Cavalcanti, Cristiane Hatsuko Baggio,Cristina Setim Freitas. Volume 107, Issue 2, 19 September 2006, Pages 277–284. 9. Achillea millefolium L. s.l. – Is the anti-inflammatory activity mediated by protease inhibition? Birgit Benedek, Brigitte Kopp, Matthias F. Melzig. Volume 113, Issue 2, 5 September 2007, Pages 312–317 | |
Yellow Dock Root
Broad-Leaved Dock, Curled Dock, Curly Dock, Field Sorrel, Yellowdock. Scientific Name: Rumex crispus. Family: Polygonaceae. People Use This For: Yellow dock is used for acute and chronic inflammation of nasal passages and the respiratory tract, as an adjunct to antibacterial therapy, a laxative, tonic. Historically, yellow dock has been used for chronic skin diseases, dermatitis, rashes, scurvy, obstructive jaundice, and psoriasis with constipation. Safety: No concerns regarding safety. No reports of harm when used therapeutically.
Pregnancy and Lactation: Refer to a Medical Herbalist. Effectiveness: There is insufficient scientific information available about the effectiveness of yellow dock. Mechanism of Action: The applicable parts of yellow dock are the root and rhizome. Yellow dock contains anthraquinone glycosides, and tannins.56,57,58,59 Oxalate content is high in the leaves and low in the stalks.60 The anthroquinones (2-4%) have a mild stimulant laxative effect.56,58,59 Anthroid laxative use is not associated with an increased risk of developing colorectal adenoma or carcinoma.61 The tannins (12 20%)59,62 are responsible for the astringent effect.57 Yellow dock is reported to stimulate bile production.56 The leaves of yellow dock contain provitamin A (beta-carotene) and iron.62 Adverse Reactions: No reported problems with therapeutic amounts of root. Interactions with Herbs & Supplements: No reported problems with therapeutic amounts of root. Interactions with Drugs: No reported problems with therapeutic amounts of root. Interactions with Foods: No reported problems with therapeutic amounts of root. Interactions with Lab Tests: No reported problems with therapeutic amounts of root. Interactions with Diseases or Conditions: No reported problems with therapeutic amounts of root.
Dosage/Administration: Dr Clare’s Blends: 1gm per day
Oral: Typical dose of the dried root is 2-4 grams or as a tea.56 The liquid extract and a tincture 1-2 mL (1:5 in 45% alcohol) have also been used.56 Specific References: YELLOW DOCK 56. Newall CA, Anderson LA, Philpson JD. Herbal Medicine: A Guide for Healthcare Professionals. London, UK: The Pharmaceutical Press, 1996. 57. Foster S, Tyler VE. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 3rd ed., Binghamton, NY: Haworth Herbal Press, 1993. 58. The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 59. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997. 60. Ellenhorn MJ, et al. Ellenhorn's Medical Toxicology: Diagnoses and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams & Wilkins, 1997. 61 Nusko G, Schneider B, Schneider I, et al. Anthranoid laxative use is not a risk factor for colorectal neoplasia: results of a prospective case control study. Gut 2000;46:651-5. 62. Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications, 1998.
| |


 Also known as
Also known as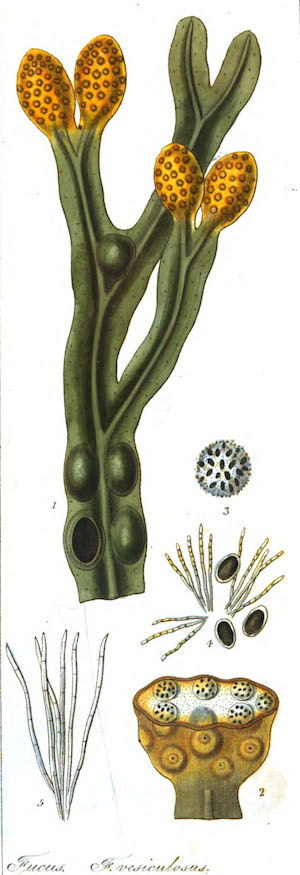 Also Known As: Fucus, Kelp, Knotted Wrack.
Also Known As: Fucus, Kelp, Knotted Wrack. Also Known As:
Also Known As: Also Known As:
Also Known As: Boswellia
Boswellia Also Known As:
Also Known As: Also Known As:
Also Known As: 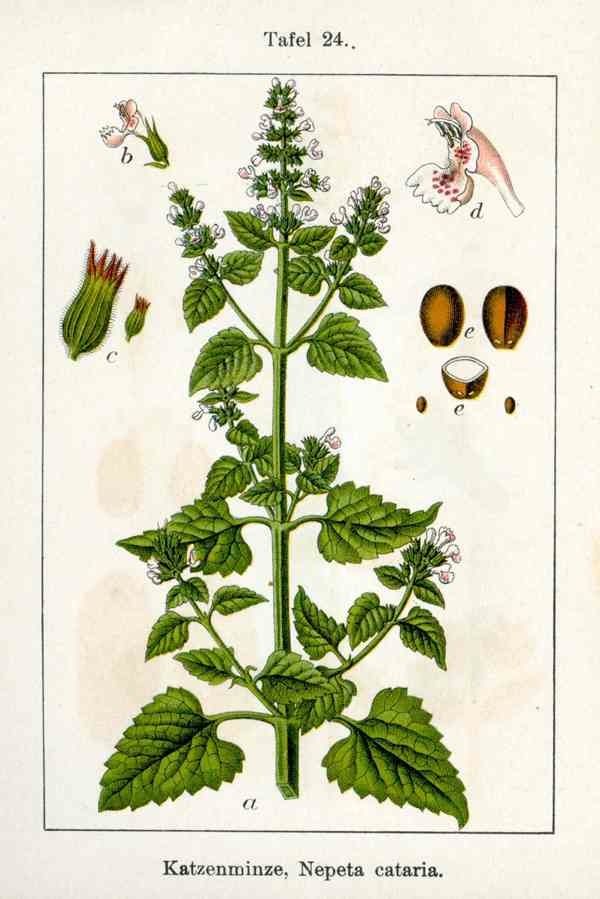 Also Known As:
Also Known As: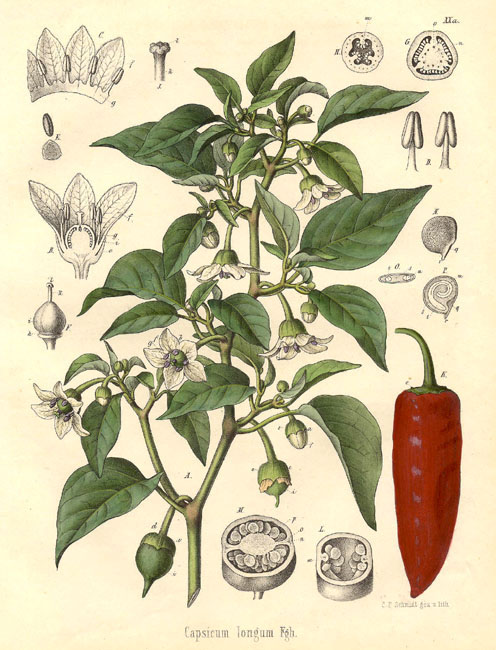 Also Known As:
Also Known As: Also Known As:
Also Known As:
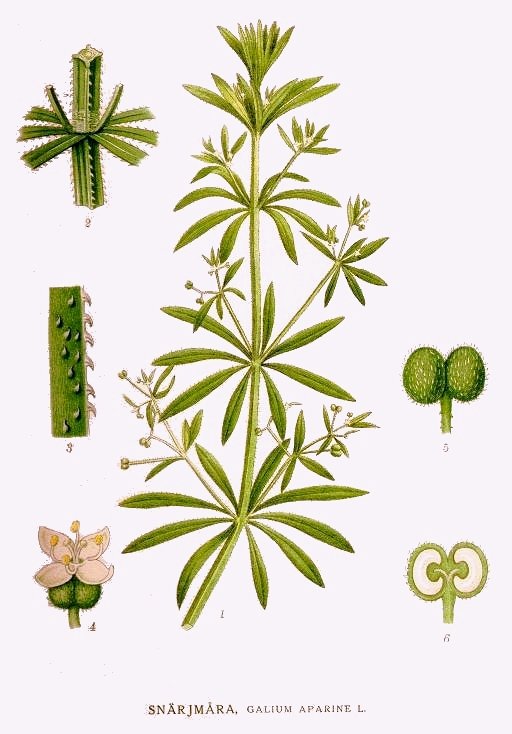 Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Cramp Bark
Cramp Bark Dandelion Root
Dandelion Root The botanical name Harpagophytum means ‘hook plant’ in Greek, it is named after the hook-covered fruits of the plant. Devil’s claw is native to southern Africa and has been used traditionally as a bitter tonic for digestive disturbances, febrile illnesses, allergic reactions and to relieve pain.
The botanical name Harpagophytum means ‘hook plant’ in Greek, it is named after the hook-covered fruits of the plant. Devil’s claw is native to southern Africa and has been used traditionally as a bitter tonic for digestive disturbances, febrile illnesses, allergic reactions and to relieve pain.  Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As:
 Also
Known As:
Also
Known As:  Also Known As:
Also Known As:  Also known as:
Also known as: 
 Also known as: Green Sencha Tea, Japanese Tea.
Also known as: Green Sencha Tea, Japanese Tea. Also Known As:
Also Known As:  Also
Known As: Bottle Brush, Common Horsetail, Corn Horsetail, Dutch Rushes,
Equiseti Herba, Field Horsetail, Herbe à Récurer, Horse Herb, Horse Willow,
Paddock-Pipes, Pewterwort, Scouring Rush, Shave Grass, Spring Horsetail, Toadpipe.
Also
Known As: Bottle Brush, Common Horsetail, Corn Horsetail, Dutch Rushes,
Equiseti Herba, Field Horsetail, Herbe à Récurer, Horse Herb, Horse Willow,
Paddock-Pipes, Pewterwort, Scouring Rush, Shave Grass, Spring Horsetail, Toadpipe. Also Known As:
Also Known As: 
 Also Known As:
Also Known As:  Also Known As:
Also Known As:  Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also known as: Bridewort, Dropwort, Filipendula, Lady of the Meadow, Meadow Queen, Meadow-Wort, Queen of the Meadow, Spiraeae Flos, Spireae Herba.
Also known as: Bridewort, Dropwort, Filipendula, Lady of the Meadow, Meadow Queen, Meadow-Wort, Queen of the Meadow, Spiraeae Flos, Spireae Herba. Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also known as: Black Peppermint, Herba Menthae, Lamb Mint, Mentha Piperita.
Also known as: Black Peppermint, Herba Menthae, Lamb Mint, Mentha Piperita. Also known as: Angelica Tree, Northern Prickly Ash, Toothache Bark, Xanthoxylum, Yellow Wood, Zanthoxylum.
Also known as: Angelica Tree, Northern Prickly Ash, Toothache Bark, Xanthoxylum, Yellow Wood, Zanthoxylum. Also Known As:
Also Known As: Also known as:Meadow Clover, Trifolium, Wild Clover.
Also known as:Meadow Clover, Trifolium, Wild Clover.  Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also known as:Betula, Betulae folium, Silver Birch, White Birch.
Also known as:Betula, Betulae folium, Silver Birch, White Birch. Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also Known As:
Also Known As: Also known as:Achillea, Carpenter's Herb, Common Yarrow, Nosebleed, Soldier's Wound Wort, Knight’s milfoil, Bloodwort, Sanguinary.
Also known as:Achillea, Carpenter's Herb, Common Yarrow, Nosebleed, Soldier's Wound Wort, Knight’s milfoil, Bloodwort, Sanguinary.